Abstract
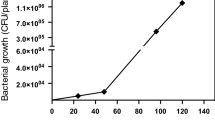
Oats produce a group of secondary metabolites termed avenanthramides (avn). These compounds are biosynthesized through the action of the enzyme hydroxycinnamoyl CoA: hydroxyanthranilate N-hydroxycinnamoyl transferase (HHT) which catalyzes the condensation of one of several cinnamate CoA thioesters with the amine functionality of anthranilic acid, 4-hydroxy- or 5-hydroxy-anthranilic acid. In oat leaf tissue the biosynthesis of avenanthramides appears to result from elicitation by fungal infection. Here we demonstrate the biosynthesis of several avenanthramides in suspension cultures of oat apical meristem callus tissue. This phenomenon appears as a generalized pathogen response, evidenced by the production of PR-1 mRNA, in response to elicitation with chitin (poly-N-acetyl glucosamine). The suspension cultures also produce relatively large quantities of avnA and G in response to chitin elicitation. Under certain culture conditions avnB and C are also produced as well as three additional metabolites tentatively identified as avnH, O and R. These findings portend the utility of oat suspension culture as a tool for more detailed investigation of the mechanisms triggering their biosynthesis as well as the factors dictating the particular types of avenanthramides biosynthesized.






Similar content being viewed by others
References
Blaakmeer A, Stork A, van Veldhuizen A, van Beek TA, de Groot A, van Loon JJA, Schoonhoven LM (1994a) Isolation, identification, and synthesis of miriamides, new hostmarkers from eggs of Pieris brassicae. J Nat Prod 57:90–99. doi:10.1021/np50103a013
Blaakmeer A, van der Wal D, Stork A, van Beek TA, de Groot A (1994b) Structure activity relationship of isolated avenanthramide alkaloids and synthesized related compounds as oviposition deterrents for Pieris brassicae. J Nat Prod 57:1145–1151. doi:10.1021/np50110a003
Bratt K, Sunnerheim K, Bryngelsson S, Fagerlund A, Engman L, Andersson RE, Dimberg LH (2003) Avenanthramides in oats (Avena sativa L.) and structure-antioxidant activity relationships. J Agric Food Chem 51:594–600. doi:10.1021/jf020544f
Collins FW (1989) Oat phenolics: avenanthramides, novel substituted N-cinnamoylanthranilate alkaloids from oat groats and hulls. J Agric Food Chem 37:60–66. doi:10.1021/jf00085a015
Collins FW, Mullin WJ (1988) High-performance liquid chromatographic determination of avenanthramides, n-aroylanthranilic acid alkaloids from oats. J Chromatogr A 445:363–370. doi:10.1016/S0021-9673(01)84548-9
Collins FW, Paton D (1992) Methods of producing stable bran and flour products from cereal grains. US patent 5,169,660
Dimberg LH, Theander O, Lingnert H (1993) Avenanthramides—a group of phenolic antioxidants in oats. Cereal Chem 70:637–641
Dimberg LH, Molteberg EL, Solheim R, Frolich W (1996) Variation in oat groats due to variety, storage and heat treatment. I: Phenolic compounds. J Cereal Sci 24:263–272. doi:10.1006/jcrs.1996.0058
Dimberg LH, Sunnerheim K, Sundberg B, Walsh K (2001) Stability of oat avenanthramides. Cereal Chem 78:278–281. doi:10.1094/CCHEM.2001.78.3.278
Emmons CL, Peterson DM (2001) Antioxidant activity and phenolic content of oat as affected by cultivar and location. Crop Sci 41:1676–1681
Emmons CL, Peterson DM, Paul GL (1999) Antioxidant capacity of oat (Avena sativa L.) extracts. 2. In vitro antioxidant activity and contents of phenolic and tocol antioxidants. J Agric Food Chem 47:4894–4898. doi:10.1021/jf990530i
Federico ML, Kaeppler HF, Skadsen R (2005) The complex development expression of a novel stress-responsive barley Ltp gene is determined by a shortened promoter sequence. Plant Mol Biol 57:35–51. doi:10.1007/s11103-004-6769-0
Federico M, Iñiuez-Luy F, Skadsen R, Kaeppler HF (2006) Spatial and temporal divergence of expression in duplicated barley germin-like protein-encoding genes. Genetics 174:179–190. doi:10.1534/genetics.106.058156
Forsberg RA, Kaeppler HF, Duerst RD (1999) Registration of”Belle” oat. Crop Sci 39:878–879
Ishihara A, Matsukawa T, Miyagawa H, Ueno T, Mayama S, Iwamura H (1997) Induction of hydroxycinnamoyl-CoA:hydroxyanthranilate N-hydroxycinnamoyl transferase (HHT) activity in oat leaves by victorin C. Z. Naturforsch. C J Biosci 52c:756–760
Ishihara A, Miyagawa H, Matsukawa T, Ueno T, Mayama S, Iwamura H (1998) Induction of hydroxyanthranilate hydroxycinnamoyl transferase by oligo-N-acetylchitooligosaccharides in oats. Phytochemistry 47:969–974. doi:10.1016/S0031-9422(97)00603-1
Ishihara A, Ohtsu Y, Iwamura H (1999a) Biosynthesis of oat avenanthramide phytoalexins. Phytochemistry 50:237–242. doi:10.1016/S0031-9422(98)00535-4
Ishihara A, Ohtsu Y, Iwamura H (1999b) Induction of biosynthetic enzymes for avenanthramides in elicitor-treated oat leaves. Planta 208:512–518. doi:10.1007/s004250050588
Ji LL, Lay D, Chung E, Fu Y, Peterson DM (2003) Effects of avenanthramides on oxidant generation and antioxidant enzyme activity in exercised rats. Nutr Res 23:1579–1590. doi:10.1016/S0271-5317(03)00165-9
Lawton K, Weymann K, Friedrich L, Vernooij B, Uknes S, Ryals J (1995) Systemic acquired resistance in Arabidopsis requires salicylic acid but not ethylene. Mol Plant Microbe Interact 8:863–870
Liu L, Zubik L, Collins FW, Marko M, Meydani M (2004) The antiatherogenic potential of oat phenolic compounds. Atherosclerosis 175:39–49. doi:10.1016/j.atherosclerosis.2004.01.044
Malamy J, Carr JP, Klessig DF, Raskin I (1990) Salicylic acid: a likely endogenous signal in the resistance response of tobacco to viral infection. Science 250:1002–1004. doi:10.1126/science.250.4983.1002
Mayama S, Matsuura Y, Inda H, Tani T (1982) The role of avenalumin in the resistance of oat to crown rust, Puccinia coronata f. sp avenae. Physiol Plant Pathol 20:189–199. doi:10.1016/0048-4059(82)90084-4
Miyagawa H, Ishihara A, Nishimoto T, Ueda T, Mayama S (1995) Induction of avenanthramides in oat leaves inoculated with crown rust fungus, Puccinia coronata f. sp avenae. Biosci Biotechnol Biochem 12:2305–2306
Miyagawa H, Ishihara A, Kuwahara Y, Ueno T, Mayama S (1996a) Comparative studies of elicitors that induce phytoalexin in oats. J Pestic Sci 21:203–207
Miyagawa H, Ishihara A, Kuwahara Y, Ueno T, Mayama S (1996b) A stress compound in oats induced by Victorin, a host-specific toxin from Helminthosporium victoriae. Phytochemistry 41:1473–1475. doi:10.1016/0031-9422(95)00805-5
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Nie L, Wise M, Peterson D, Meydani M (2006a) Mechanism by which avenanthramide-c, a polyphenol of oats, blocks cell cycle progression in vascular smooth muscle cells. Free Radic Biol Med 41:702–708. doi:10.1016/j.freeradbiomed.2006.04.020
Nie L, Wise ML, Peterson DM, Meydani M (2006b) Avenanthramide, a polyphenol from oats, inhibits vascular smooth muscle cell proliferation and enhances nitric oxide production. Atherosclerosis 186:260–266. doi:10.1016/j.atherosclerosis.2005.07.027
Okazaki Y, Isobe T, Iwata Y, Matsukawa T, Matsuda F, Miyagawa H, Ishihara A, Nishioka T, Iwamura H (2004) Metabolism of avenanthramide phytoalexins in oats. Plant J 39:560–572. doi:10.1111/j.1365-313X.2004.02163.x
Okazaki Y, Ishizuka A, Ishihara A, Nishioka T, Iwamura H (2007) New dimeric compounds of avenanthramide phytoalexin in oats. J Org Chem 72:3830–3839
Peterson DM, Hahn MJ, Emmons CL (2002) Oat avenanthramides exhibit antioxidant activities in vitro. Food Chem 79:473–478. doi:10.1016/S0308-8146(02)00219-4
Ponchet M, Favre-Bonvin J, Hauteville M, Ricci P (1988) Dianthramides (N-benzoyl and N-paracoumarylanthranilic acid derivatives) from elicited tissues of Dianthus caryophyllus. Phytochemistry 27:725–730. doi:10.1016/0031-9422(88)84083-4
Ren Y-Y, West CA (1992) Elicitation of diterpene biosynthesis in rice (Oryza sativa L.) by chitin. Plant Physiol 99:1169–1178
Ryan CA (1987) Oligosaccharide signalling in plants. Annu Rev Cell Biol 3:295–317. doi:10.1146/annurev.cb.03.110187.001455
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Spoel SH, Koornneef A, Claessens SMC, Korzelius JP, Van Pelt JA, Mueller MJ, Buchala AJ, Metraux J-P, Brown R, Kazan K, Van Loon LC, Dong X, Pieterse CMJ (2003) NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 15:760–770. doi:10.1105/tpc.009159
Stockgit J, Zenk MH (1975) Chemical synthesis and properties of hydroxycinnamoyl-coenzyme A derivatives. Z Naturforsch [C] 30:352–358
van Loon LC, Rep M, Pieterse CM (2006) Significance of inducible defense-related proteins in infected plants. Annu Rev Phytopathol 44:135–162
Vollhardt J, Fielder DA, Redmond MJ (2000) Identification and cosmetic application of powerful anti-irritant constituents of oat grain. XXIst IFSCC International Congress, Berlin
Yang Q, Trinh HX, Imai S, Ishihara A, Zhang L, Nakayashiki H, Tosa Y, Mayama S (2004) Analysis of the involvement of hydroxyanthranilate hydroxycinnamoyltransferase and caffeoyl-CoA 3-O-methyltransferase in phytoalexin biosynthesis in oat. Mol Plant Microb Interact 17:81–89
Acknowledgments
Thanks are extended to Rachel Angel, Laurie Herrin, John Herbst, and Nick Partridge for expert technical assistance. NMR data was obtained from the National Magnetic Resonance Facility at Madison, which is supported by NIH grants P41RR02301 (BRTP/NCRR) and P41GM66326 (NIGMS). Additional equipment was purchased with funds from the University of Wisconsin, the NIH (RR02781, RR08438), the NSF (DMB-8415048, OIA-9977486, BIR-9214394), and the USDA. This research is supported by the USDA, Agricultural Research Service CRIS #3655-21000-044-00D.
Disclaimer
Names are necessary to report factually on available data; however, the USDA neither guarantees nor warrants the standard of the product, and the use of he name by USDA implies no approval of the product to the exclusion of others that may also be suitable.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wise, M.L., Sreenath, H.K., Skadsen, R.W. et al. Biosynthesis of avenanthramides in suspension cultures of oat (Avena sativa). Plant Cell Tiss Organ Cult 97, 81–90 (2009). https://doi.org/10.1007/s11240-009-9501-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-009-9501-6