Abstract
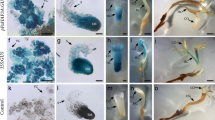
Capsicum chinense is a recalcitrant species for in vitro morphogenesis, and up to date there is no efficient system for genetic transformation and regeneration of this species via somatic embryogenesis. Here, we carried out an in vitro transformation of C. chinense via Agrobacterium tumefaciens co-cultivation with a system that expresses the heterologous gene WUSCHEL from Arabidopsis thaliana. WUSCHEL has been shown to promote the transition from vegetative to embryogenic state when overexpressed. We tested if the expression of WUSCHEL in C. chinense would promote an embryogenic response in this species. After 15 days of induction, the segments of transformed stems begun to form globular structures, suggesting that heterologus WUSCHEL was active and involved in the process of morphogenesis.




Similar content being viewed by others
Abbreviations
- BAP:
-
6-Benzylaminopurine
- IAA:
-
Indoleacetic acid
- NAA:
-
Naphthaleneacetic acid
- MS:
-
Murashige and Skoog
- PCR:
-
Polymerase chain reaction
- SDS:
-
Sodium dodecyl sulfate
- 3R:
-
BAP + IAA + NAA
References
Arroyo-Herrera A, Ku-González A, Canche-Moo R, Quiróz-Figueroa FR, Loyola Vargas V, Rodríguez Zapata LC, Burgeff D’Hondt C, Suárez-Solís VM, Castaño E (2008) Expression of WUSCHEL in Coffea canephora causes ectopic morphogenesis and increases somatic embryogenesis. Plant Cell Tissue Organ Cult 94:171–180. doi:10.1007/s11240-008-9401-1
Binzel ML, Sankhla N, Joshi S, Sankhla D (1996) Induction of direct somatic embryogenesis and plant regeneration in pepper (Capsicum annuum L.). Plant Cell Rep 15:536–540. doi:10.1007/BF00232989
Buyukalaca S, Mavituna F (1996) Somatic embryogenesis and plant regeneration of pepper in liquid media. Plant Cell Tissue Organ Cult 46:227–235. doi:10.1007/BF02307099
Cai W, Rong-Xiang F, Feng-Li Z, Jiu-Chun Z, Xiaoying CH, Gui-Ling W, Ke-Qiang M, Hong-Sheng S, Xu W, Yue-Ren l (2002) Virus-resistant Chili pepper produced by Agrobacterium speciesmediated transformation. In: Khachatourians G, Hughen Mc, Scorza R, Nip WK, Hui YH (eds) Transgenic plants and crops. Marcel Dekker, Inc., NY, pp 563–577
Cai WQ, Fang X, Shang HS, Wang X, Mang KQ (2003) Development of CMV- and TMV- resistant transgenic chilli pepper: field performance and biosafety assessment. Mol Breed 11:25–35. doi:10.1023/A:1022655204552
Canche-Moo R, Ku-Gonzalez A, Burgeff C, Loyola-Vargas V, Rodriguez-Zapata LC, Castaño E (2006) Genetic transformation of Coffea canephora by vacuum infiltration. Plant Cell Tissue Organ Cult 84:373–377. doi:10.1007/s11240-005-9036-4
Dabauza M, Peña l (2003) Response of sweet pepper (Capsicum annuum L.) genotypes to Agrobacterium tumefaciens as a means of selecting proper vectors for genetic transformation. J Hortic Sci Biotechnol 78:65–72
Gallie DR, Lucas WJ, Walbot V (1989) Visualizing mRNA expression in plant protoplasts: factors influencing efficient mRNA uptake and translation. Plant Cell 1:303–311
Gallois JL, Woodward C, Reddy GV, Sablowski R (2002) Combined SHOOT MERISTEMLESS and WUSCHEL trigger ectopic organogenesis in Arabidopsis. Development 129:3207–3217
Gallois JL, Nora FR, Mizukami Y, Sablowski R (2004) WUSCHEL induces shoot stem cell activity and developmental plasticity in the root meristem. Genes Dev 18:375–380. doi:10.1101/gad.291204
Herrera-Estrella L, Simpson J, Martínez-Trujillo M (2004) Transgenic plants. An historical perspective. In: Peña L (ed) Transgenic plants methods and protocols, vol 286. Humana Press, Totowa, NJ, pp 3–31
Lee HY, Kim HS, Kim JY, Jung M, Park YS, Lee JS, Choi SH, Her NH, Lee JH, Hyung NI, Lee CH, Yang SG, Harn CH (2004) A new selection meted for pepper transformation: callus-mediated shoot formation. Genetic Transformation and Hibridization. Plant Cell Rep 23:50–58
Li D, Zhao K, Xie B, Zhang B, Luo K (2003) Establishment of a highly efficient transformation system for pepper (Capsicum annuum L.). Plant Cell Rep 21:785–788. doi:10.1007/s00299-003-0581-1
Liu W, Parrot WA, Hildebrand DF, Collins GB, Williams EG (1990) Agrobacterium induced gall formation in bell pepper (Capsicum annuum L.) and formation of shoot-like structures expressing introduced genes. Plant Cell Rep 9:360–364
López-Puc G, Canto-Flick A, Barredo-Pool F, Zapata-Castillo P, Peniche-Montalvo M, Barahona-Pérez F, Iglesias-Andreu l, Santana-Buzzy N (2006) Direct somatic embryogenesis: a highly efficient protocol for in vitro regeneration of habanero pepper (Capscium chinense Jacq.). HortScience 41(7):1645–1650
Manoharan M, Sree CS, Lakshmi S (1998) Agrobacterium mediated genetic transformation in hot chilli (Capsicum annuum L. var Pusa jwala). Plant Science 131:77–83 PII S0168-9452(97)00231-8
Mayer KF, Schoof H, Haecker A, Lenhard M, Jurgens G, Laux T (1998) Role of WUSCHEL in regulating stem cell fate in Arabidopsis shoot meristem. Cell 95:805–815. doi:10.1016/S0092-8674(00)81703-1
Mihalka V, Fari M, Szasz A, Balazs E, Nagy I (2000) Optimised protocols for efficient plant regeneration and gene transfer in pepper (Capsicum annum L.). J Plant Biotechnol 2(3):143–149
Mihalka V, Balazs E, Nagy I (2003) Binary transformation systems based on “shooter” mutants of Agrobacterium tumefaciens. A simple, efficient and universal gene transfer technology that permits marker gene elimination. Plant Cell Rep 21:778–784. doi:10.1007/s00299-003-0597-6
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497. doi:10.1111/j.1399-3054.1962.tb08052.x
Noriko K, Hiroshi N, Atsushi M, Yutaka S, Makoto M (2003) Isolation and characterization of a rice WUSCHEL-type homeobox gene that is specifically expressed in the central cells of a quiescent center in the root apical meristem. Plant J 35:429–441. doi:10.1046/j.1365-313X.2003.01816.x
Ochoa-Alejo N, Ramírez-Malagon R (2001) Invited review. In vitro chilli pepper biotechnology. In Vitro Cell Dev Biol 37:701–709. doi:10.1079/IVP2001216
Romero-Pozueta J, Houlne G, Cañas L, Schantz R, Chamarro J (2001) Enhanced regeneration of tomato and pepper seedling plants explants for Agrobacterium-mediated transformation. Plant Cell Tissue Organ Cult 67:173–180. doi:10.1023/A:1011997926381
Santana-Buzzy N, Canto-Flick A, Barahona-Pérez F, Montalvo-Peniche MC, Zapata-Castillo P, Solís-Ruíz A, Zaldívar-Collí A, Gutiérrez-Alonso O, Miranda-Ham L (2005) Regeneration of habanero pepper (Capsicum chinense Jacq) via organogenesis. HortScience 40(6):1829–1831
Shin R, Han JH, Lee GJ, Peak KH (2002a) The potencial use of viral coat protein genes as transgene screening marker and multiple virus resistance of pepper plants coexpressing coat proteins of cucumber mosaic virus and tomato mosaic virus. Transgenic Res 11:215–219. doi:10.1023/A:1015200622716
Shin R, Park J, An JM, Paek KH (2002b) Ectopic expression of Tsi1 in transgenic hot pepper plants enhaces host resistance to viral, bacterial, and oomycete pahogens. The American Phytopathological Society. MPMI, vol 15(10), pp 983–989, no.M-2002-0812-02R
Shivegowda T, Mythili JB, Anand l, Saipradad G, Ramanjini Gowda , Gowda TK (2002) In vitro regeneration and transformation in chilli pepper (Capsicum annuum L.). J Hortic Sci Biotechnol 77(5):629–634
Venkataiah P, Christopher T, Subhash K (2001) Plant regeneration and Agrobacterium-mediated genetic transformation in tour Capsicum species. Capsicum Eggplant Newslett 20:68–71
Yu-Xian X, Wen-Jun OY, Yi-Feng Z, Zhang-Liang CH (1996) Transgenic sweet pepper plants from Agrobacterium mediated transformation. Plant Cell Rep 16:71–75. doi:10.1007/BF01275453
Zhu Y, Ou-Yang WJ, Zhang YF, Chen ZL (1996) Transgenic sweet pepper plants from Agrobacterium mediated transformation. Plant Cell Rep 16:71–75. doi:10.1007/BF01275453
Zuo J, Niu QW, Chua NH (2000) An estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J 24(2):265–273. doi:10.1046/j.1365-313x.2000.00868.x
Zuo J, Niu QW, Frugis G, Chua NH (2002) The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J 30(3):349–359. doi:10.1046/j.1365-313X.2002.01289.x
Acknowledgments
We like to thank Dirección de Intercambio Academico de la Secretaria de Relaciones Exteriores from Mexico and Centro de Investigaciones Cientificas de Yucatan. Also the tecnical help of Angela Ku Gonzalez.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Solís-Ramos, L.Y., González-Estrada, T., Nahuath-Dzib, S. et al. Overexpression of WUSCHEL in C. chinense causes ectopic morphogenesis. Plant Cell Tiss Organ Cult 96, 279–287 (2009). https://doi.org/10.1007/s11240-008-9485-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9485-7