Abstract
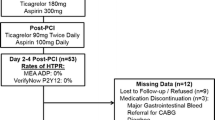
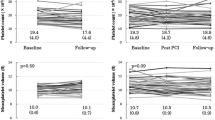
Prolonged use of dual antiplatelet therapy (DAPT) post-percutaneous coronary intervention (PCI) has been shown to reduce the risk of major adverse cardiovascular events (MACE), but with increased bleeding. It remains unknown whether biomarkers of platelet activation may be useful for identifying patients at increased risk of MACE. The DAPT study was a randomized trial of 12 versus 30 months of DAPT in patients who underwent PCI. Serum biomarkers [myeloid-related protein (MRP)-8/14, P-selectin, soluble CD-40 ligand (sCD40L)] were assessed in 1399 patients early post-PCI. On-treatment platelet reactivity index (PRI) using VASP phosphorylation was assessed in 443 patients randomized to continued DAPT at 1 year. MACE was defined as CV death, MI, or ischemic stroke. Multivariable models were adjusted for baseline characteristics, index event, and stent type. A stepwise increase in the risk of MACE was observed with increasing tertiles of both MRP-8/14 and P-selectin (p-trend = 0.04 for both). After multivariable adjustment, the adjusted HR (95% CI) for MACE in patients in the top tertile was 1.94 (1.14–3.30) for MRP-8/14 and 1.62 (0.99–2.64) for P-selectin. In contrast, baseline sCD40L was not associated with CV risk. Among patients randomized to continued DAPT, higher on-treatment platelet reactivity was not significantly associated with risk of MACE (p-trend = 0.32; adj-HR T3 vs. T1 1.54, 95% CI 0.20–12.18) or bleeding (P-trend = 0.17; adj-HR 0.25, 95% CI 0.05–1.21). MRP-8/14 and soluble P-selectin may be useful for identifying patients at increased risk of MACE after PCI. The utility of on-treatment platelet function testing requires further study.
Clinical Trial Registration https://www.clinicaltrials.gov. Unique identifier NCT00977938.


Similar content being viewed by others
Data availability
Data requests can be made to Baim Research Institute.
Code availability
Data requests can be made to Baim Research Institute.
References
Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS, American Heart Association Council on Epidemiology, Prevention Statistics Committee, Stroke Statistics Subcommittee (2019) Heart disease and stroke statistics—2019 update: a Report from the American Heart Association. Circulation 139(10):e56–e528. https://doi.org/10.1161/CIR.0000000000000659
Amsterdam EA, Wenger NK, Brindis RG, Casey DE Jr, Ganiats TG, Holmes DR Jr, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ, AATF Members (2014) 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 130(25):e344–e426. https://doi.org/10.1161/CIR.0000000000000134
Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM, Khot UN, Lange RA, Mauri L, Mehran R, Moussa ID, Mukherjee D, Ting HH, O’Gara PT, Kushner FG, Ascheim DD, Brindis RG, Casey DE Jr, Chung MK, de Lemos JA, Diercks DB, Fang JC, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX (2016) 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 133(11):1135–1147. https://doi.org/10.1161/CIR.0000000000000336
Mauri L, Kereiakes DJ, Yeh RW, Driscoll-Shempp P, Cutlip DE, Steg PG, Normand SL, Braunwald E, Wiviott SD, Cohen DJ, Holmes DR Jr, Krucoff MW, Hermiller J, Dauerman HL, Simon DI, Kandzari DE, Garratt KN, Lee DP, Pow TK, Ver Lee P, Rinaldi MJ, Massaro JM, DS Investigators (2014) Twelve or 30 months of dual antiplatelet therapy after drug-eluting stents. N Engl J Med 371(23):2155–2166. https://doi.org/10.1056/NEJMoa1409312
Yeh RW, Secemsky EA, Kereiakes DJ, Normand SL, Gershlick AH, Cohen DJ, Spertus JA, Steg PG, Cutlip DE, Rinaldi MJ, Camenzind E, Wijns W, Apruzzese PK, Song Y, Massaro JM, Mauri L, DS Investigators (2016) Development and validation of a prediction rule for benefit and harm of dual antiplatelet therapy beyond 1 year after percutaneous coronary intervention. JAMA 315(16):1735–1749. https://doi.org/10.1001/jama.2016.3775
Viemann D, Strey A, Janning A, Jurk K, Klimmek K, Vogl T, Hirono K, Ichida F, Foell D, Kehrel B, Gerke V, Sorg C, Roth J (2005) Myeloid-related proteins 8 and 14 induce a specific inflammatory response in human microvascular endothelial cells. Blood 105(7):2955–2962. https://doi.org/10.1182/blood-2004-07-2520
Vogl T, Ludwig S, Goebeler M, Strey A, Thorey IS, Reichelt R, Foell D, Gerke V, Manitz MP, Nacken W, Werner S, Sorg C, Roth J (2004) MRP8 and MRP14 control microtubule reorganization during transendothelial migration of phagocytes. Blood 104(13):4260–4268. https://doi.org/10.1182/blood-2004-02-0446
Healy AM, Pickard MD, Pradhan AD, Wang Y, Chen Z, Croce K, Sakuma M, Shi C, Zago AC, Garasic J, Damokosh AI, Dowie TL, Poisson L, Lillie J, Libby P, Ridker PM, Simon DI (2006) Platelet expression profiling and clinical validation of myeloid-related protein-14 as a novel determinant of cardiovascular events. Circulation 113(19):2278–2284
Michelson AD, Barnard MR, Hechtman HB, MacGregor H, Connolly RJ, Loscalzo J, Valeri CR (1996) In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci U S A 93(21):11877–11882. https://doi.org/10.1073/pnas.93.21.11877
Fijnheer R, Frijns CJ, Korteweg J, Rommes H, Peters JH, Sixma JJ, Nieuwenhuis HK (1997) The origin of P-selectin as a circulating plasma protein. Thromb Haemost 77(6):1081–1085
Ridker PM, Buring JE, Rifai N (2001) Soluble P-selectin and the risk of future cardiovascular events. Circulation 103(4):491–495. https://doi.org/10.1161/01.cir.103.4.491
Blann AD, Faragher EB, McCollum CN (1997) Increased soluble P-selectin following myocardial infarction: a new marker for the progression of atherosclerosis. Blood Coagul Fibrinolysis 8(7):383–390
Heeschen C, Dimmeler S, Hamm CW, van den Brand MJ, Boersma E, Zeiher AM, Simoons ML, CS Investigators (2003) Soluble CD40 ligand in acute coronary syndromes. N Engl J Med 348(12):1104–1111. https://doi.org/10.1056/NEJMoa022600
Schwarz UR, Geiger J, Walter U, Eigenthaler M (1999) Flow cytometry analysis of intracellular VASP phosphorylation for the assessment of activating and inhibitory signal transduction pathways in human platelets—definition and detection of ticlopidine/clopidogrel effects. Thromb Haemost 82(3):1145–1152
Frere C, Cuisset T, Quilici J, Camoin L, Carvajal J, Morange PE, Lambert M, Juhan-Vague I, Bonnet JL, Alessi MC (2007) ADP-induced platelet aggregation and platelet reactivity index VASP are good predictive markers for clinical outcomes in non-ST elevation acute coronary syndrome. Thromb Haemost 98(4):838–843
Cuisset T, Frere C, Quilici J, Morange PE, Nait-Saidi L, Carvajal J, Lehmann A, Lambert M, Bonnet JL, Alessi MC (2006) Benefit of a 600-mg loading dose of clopidogrel on platelet reactivity and clinical outcomes in patients with non-ST-segment elevation acute coronary syndrome undergoing coronary stenting. J Am Coll Cardiol 48(7):1339–1345. https://doi.org/10.1016/j.jacc.2006.06.049
Mehran R, Rao SV, Bhatt DL, Gibson CM, Caixeta A, Eikelboom J, Kaul S, Wiviott SD, Menon V, Nikolsky E, Serebruany V, Valgimigli M, Vranckx P, Taggart D, Sabik JF, Cutlip DE, Krucoff MW, Ohman EM, Steg PG, White H (2011) Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation 123(23):2736–2747. https://doi.org/10.1161/CIRCULATIONAHA.110.009449
Olenchock BA, Wiviott SD, Murphy SA, Cannon CP, Rifai N, Braunwald E, Morrow DA (2008) Lack of association between soluble CD40L and risk in a large cohort of patients with acute coronary syndrome in OPUS TIMI-16. J Thromb Thrombolysis 26(2):79–84. https://doi.org/10.1007/s11239-007-0156-z
Funding
Dr. Berg is supported by a T32 postdoctoral training grant from the National Heart, Lung, and Blood Institute (T32 HL007604). Dr. Sabatine and Dr. O’Donoghue were supported by grant R01HL098082 from the National Heart, Lung, and Blood Institute for this investigation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Berg has nothing to disclose. Dr. Yeh has received Consulting Fees and Research Grants from Abbott Vascular, AstraZeneca, Boston Scientific and Medtronic. Laura Mauri is currently a Full-Time Employee of Medtronic. Dr. Morrow has received Grants and Personal Fees from Abbott Laboratories, AstraZeneca, Roche Diagnostics, and Bayer Pharma; Grants from Novartis, BRAHMS, Daiichi-Sankyo, Eisai, GlaxoSmithKline, Takeda, Pfizer, Quark, The Medicines Company, Merck, and Zora Diagnostics; Personal Fees from InCarda, Aralez, Peloton, and Verseon. He is a Member of the TIMI Study Group, for which he has received Institutional Research Grant support through Brigham and Women’s Hospital from Abbott, Amgen, Aralez, AstraZeneca, Bayer HealthCare Pharmaceuticals, Inc., BRAHMS, Daiichi Sankyo, Eisai, GlaxoSmithKline, Intarcia, Janssen, MedImmune, Merck, Novartis, Pfizer, Poxel, Quark Pharmaceuticals, Roche, Takeda, The Medicines Company, and Zora Biosciences. Dr. Cutlip has received Consulting Fees from Celonova. Ms. Gao has nothing to disclose. Dr. Jarolim has received Research Grants through his institution from Abbott Laboratories, Amgen, Inc., AstraZeneca, LP, Daiichi-Sankyo, Inc., GlaxoSmithKline, Merck and Co, Inc., Roche Diagnostics Corporation, Takeda Global Research and Development Center, and Waters Technologies Corporation; and Consulting Fees from Roche Diagnostics Corporation. Dr. Michelson has received Research Grants from Ironwood Pharmaceuticals. Dr. Frelinger has received Research Grants from Ironwood Pharmaceuticals. Dr. Cange has nothing to disclose. Dr. Sabatine reports Grants from AstraZeneca during the conduct of the study; Grants and Personal Fees from Amgen, Personal Fees from Anthos Therapeutics, Grants and Personal Fees from AstraZeneca, Grants from Bayer, Personal Fees from Bristol-Myers Squibb, Personal Fees from CVS Caremark, Grants from Daiichi-Sankyo, Personal Fees from DalCor, Personal Fees from Dyrnamix, Grants from Eisai, Personal Fees from Esperion, Grants from GlaxoSmithKline, Personal Fees from IFM Therapeutics, Grants and Personal Fees from Intarcia, Personal Fees from Ionis, Grants and Personal Fees from Janssen Research and Development, Grants and Personal Fees from Medicines Company, Grants and Personal Fees from MedImmune, Grants and Personal Fees from Merck, Grants and Personal Fees from Novartis, Grants from Pfizer, Grants from Poxel, Grants from Quark Pharmaceuticals, Grants from Takeda, outside the submitted work; and is a Member of the TIMI Study Group, which has also received Institutional Research Grant support through Brigham and Women’s Hospital from: Abbott, Aralez, Roche, and Zora Biosciences. Dr. O’Donoghue has received Research Grants from GlaxoSmithKline, Janssen, The Medicines Company, and Merck.
Ethical approval
Ethics approval was obtained for all participating institutions.
Informed consent
Informed consent was obtained for all participants. Patients were informed of intent to publish study results.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Berg, D.D., Yeh, R.W., Mauri, L. et al. Biomarkers of platelet activation and cardiovascular risk in the DAPT trial. J Thromb Thrombolysis 51, 675–681 (2021). https://doi.org/10.1007/s11239-020-02221-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-020-02221-5