Abstract
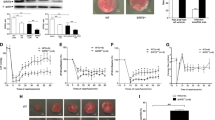
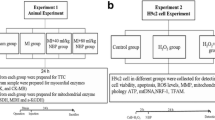
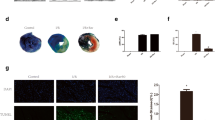
Sirtuin 3 is a nicotinamide adenine dinucleotide dependent mitochondrial deacetylase that governs mitochondrial metabolism and oxidative defense. The demise in myocardial function following myocardial ischemia has been associated with mitochondrial dysfunction. Sirt3 maintains myocardial contractile function and protects from cardiac hypertrophy. The role of Sirt3 in ischemia is controversial. Our objective was to understand, under what circumstances Sirt3 is protective in different facets of ischemia, using an in vitro proof-of-concept approach based on simulated ischemia in cultured cardiomyoblasts. Cultured H9c2 cardiomyoblasts were subjected to hypoxia and/or serum deprivation, the combination of which we refer to as simulated ischemia. Apoptosis, as assessed by Annexin V staining in life-cell imaging and propidium-iodide inclusion in flow cytometry, was enhanced following simulated ischemia. Interestingly, serum deprivation was a stronger trigger of apoptosis than hypoxia. Knockdown of Sirt3 further increased apoptosis upon serum deprivation, whereas no such effect occurred upon additional hypoxia. Similarly, only upon serum deprivation but not upon simulated ischemia, silencing of Sirt3 led to a deterioration of mitochondrial function in extracellular flux analysis. In the absence of oxygen these Sirt3-dependent effects were abolished. These data indicate, that Sirt3-mediated myocardial protection is oxygen-dependent. Thus, mitochondrial respiration takes center-stage in Sirt3-dependent prevention of stress-induced myocardial damage.




Similar content being viewed by others
References
Moran AE, Oliver JT, Mirzaie M, Forouzanfar MH, Chilov M, Anderson L, Morrison JL, Khan A, Zhang N, Haynes N, Tran J, Murphy A, Degennaro V, Roth G, Zhao D, Peer N, Pichon-Riviere A, Rubinstein A, Pogosova N, Prabhakaran D, Naghavi M, Ezzati M, Mensah GA (2012) Assessing the global burden of ischemic heart disease: part 1: methods for a systematic review of the global epidemiology of ischemic heart disease in 1990 and 2010. Glob Heart 7(4):315–329. https://doi.org/10.1016/j.gheart.2012.10.004
Ziaeian B, Fonarow GC (2016) Epidemiology and aetiology of heart failure. Nat Rev Cardiol 13(6):368–378. https://doi.org/10.1038/nrcardio.2016.25
Roes SD, Kelle S, Kaandorp TA, Kokocinski T, Poldermans D, Lamb HJ, Boersma E, van der Wall EE, Fleck E, de Roos A, Nagel E, Bax JJ (2007) Comparison of myocardial infarct size assessed with contrast-enhanced magnetic resonance imaging and left ventricular function and volumes to predict mortality in patients with healed myocardial infarction. Am J Cardiol 100(6):930–936. https://doi.org/10.1016/j.amjcard.2007.04.029
Wallace DC (2000) Mitochondrial defects in cardiomyopathy and neuromuscular disease. Am Heart J 139(2 Pt 3):S70–S85
Winnik S, Auwerx J, Sinclair DA, Matter CM (2015) Protective effects of sirtuins in cardiovascular diseases: from bench to bedside. Eur Heart J 36(48):3404–3412. https://doi.org/10.1093/eurheartj/ehv290
Chalkiadaki A, Guarente L (2012) Sirtuins mediate mammalian metabolic responses to nutrient availability. Nat Rev Endocrinol 8(5):287–296. https://doi.org/10.1038/nrendo.2011.225
Houtkooper RH, Pirinen E, Auwerx J (2012) Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol 13(4):225–238. https://doi.org/10.1038/nrm3293
Ahn BH, Kim HS, Song S, Lee IH, Liu J, Vassilopoulos A, Deng CX, Finkel T (2008) A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc Natl Acad Sci USA 105(38):14447–14452. https://doi.org/10.1073/pnas.0803790105
Sundaresan NR, Samant SA, Pillai VB, Rajamohan SB, Gupta MP (2008) SIRT3 is a stress-responsive deacetylase in cardiomyocytes that protects cells from stress-mediated cell death by deacetylation of Ku70. Mol Cell Biol 28(20):6384–6401. https://doi.org/10.1128/MCB.00426-08
Chen CJ, Fu YC, Yu W, Wang W (2013) SIRT3 protects cardiomyocytes from oxidative stress-mediated cell death by activating NF-kappaB. Biochem Biophys Res Commun 430(2):798–803. https://doi.org/10.1016/j.bbrc.2012.11.066
Cheung KG, Cole LK, Xiang B, Chen K, Ma X, Myal Y, Hatch GM, Tong Q, Dolinsky VW (2015) Sirtuin-3 (SIRT3) protein attenuates doxorubicin-induced oxidative stress and improves mitochondrial respiration in H9c2 cardiomyocytes. J Biol Chem 290(17):10981–10993. https://doi.org/10.1074/jbc.M114.607960
Zeng H, Vaka VR, He X, Booz GW, Chen JX (2015) High-fat diet induces cardiac remodelling and dysfunction: assessment of the role played by SIRT3 loss. J Cell Mol Med 19(8):1847–1856. https://doi.org/10.1111/jcmm.12556
Klishadi MS, Zarei F, Hejazian SH, Moradi A, Hemati M, Safari F (2015) Losartan protects the heart against ischemia reperfusion injury: sirtuin3 involvement. J Pharm Pharm Sci 18(1):112–123
Zeng H, Li L, Chen JX (2014) Loss of Sirt3 limits bone marrow cell-mediated angiogenesis and cardiac repair in post-myocardial infarction. PLoS ONE 9(9):e107011. https://doi.org/10.1371/journal.pone.0107011
Koentges C, Pfeil K, Schnick T, Wiese S, Dahlbock R, Cimolai MC, Meyer-Steenbuck M, Cenkerova K, Hoffmann MM, Jaeger C, Odening KE, Kammerer B, Hein L, Bode C, Bugger H (2015) SIRT3 deficiency impairs mitochondrial and contractile function in the heart. Basic Res Cardiol 110(4):36. https://doi.org/10.1007/s00395-015-0493-6
Koentges C, Pfeil K, Meyer-Steenbuck M, Lother A, Hoffmann MM, Odening KE, Hein L, Bode C, Bugger H (2016) Preserved recovery of cardiac function following ischemia-reperfusion in mice lacking SIRT3. Can J Physiol Pharmacol 94(1):72–80. https://doi.org/10.1139/cjpp-2015-0152
Bochaton T, Crola-Da-Silva C, Pillot B, Villedieu C, Ferreras L, Alam MR, Thibault H, Strina M, Gharib A, Ovize M, Baetz D (2015) Inhibition of myocardial reperfusion injury by ischemic postconditioning requires sirtuin 3-mediated deacetylation of cyclophilin D. J Mol Cell Cardiol 84:61–69. https://doi.org/10.1016/j.yjmcc.2015.03.017
Kuznetsov AV, Javadov S, Sickinger S, Frotschnig S, Grimm M (2015) H9c2 and HL-1 cells demonstrate distinct features of energy metabolism, mitochondrial function and sensitivity to hypoxia-reoxygenation. Biochim Biophys Acta 1853(2):276–284. https://doi.org/10.1016/j.bbamcr.2014.11.015
Watkins SJ, Borthwick GM, Arthur HM (2011) The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In Vitro Cell Dev Biol Anim 47(2):125–131. https://doi.org/10.1007/s11626-010-9368-1
Hescheler J, Meyer R, Plant S, Krautwurst D, Rosenthal W, Schultz G (1991) Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ Res 69(6):1476–1486
Bonavita F, Stefanelli C, Giordano E, Columbaro M, Facchini A, Bonafe F, Caldarera CM, Guarnieri C (2003) H9c2 cardiac myoblasts undergo apoptosis in a model of ischemia consisting of serum deprivation and hypoxia: inhibition by PMA. FEBS Lett 536(1–3):85–91
Walsh GM, Dewson G, Wardlaw AJ, Levi-Schaffer F, Moqbel R (1998) A comparative study of different methods for the assessment of apoptosis and necrosis in human eosinophils. J Immunol Methods 217(1–2):153–163
Charles I, Khalyfa A, Kumar DM, Krishnamoorthy RR, Roque RS, Cooper N, Agarwal N (2005) Serum deprivation induces apoptotic cell death of transformed rat retinal ganglion cells via mitochondrial signaling pathways. Invest Ophthalmol Vis Sci 46(4):1330–1338. https://doi.org/10.1167/iovs.04-0363
Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP (2009) Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest 119(9):2758–2771. https://doi.org/10.1172/JCI39162
Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV Jr, Alt FW, Kahn CR, Verdin E (2010) SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464(7285):121–125. https://doi.org/10.1038/nature08778
Stride N, Larsen S, Hey-Mogensen M, Sander K, Lund JT, Gustafsson F, Kober L, Dela F (2013) Decreased mitochondrial oxidative phosphorylation capacity in the human heart with left ventricular systolic dysfunction. Eur J Heart Fail 15(2):150–157. https://doi.org/10.1093/eurjhf/hfs172
Porter GA, Urciuoli WR, Brookes PS, Nadtochiy SM (2014) SIRT3 deficiency exacerbates ischemia-reperfusion injury: implication for aged hearts. Am J Physiol Heart Circ Physiol 306(12):H1602–H1609. https://doi.org/10.1152/ajpheart.00027.2014
Piper HM, Garcia-Dorado D, Ovize M (1998) A fresh look at reperfusion injury. Cardiovasc Res 38(2):291–300
Argaud L, Gateau-Roesch O, Raisky O, Loufouat J, Robert D, Ovize M (2005) Postconditioning inhibits mitochondrial permeability transition. Circulation 111(2):194–197. https://doi.org/10.1161/01.CIR.0000151290.04952.3B
Robich MP, Chu LM, Burgess TA, Feng J, Han Y, Nezafat R, Leber MP, Laham RJ, Manning WJ, Sellke FW (2012) Resveratrol preserves myocardial function and perfusion in remote nonischemic myocardium in a swine model of metabolic syndrome. J Am Coll Surg 215(5):681–689. https://doi.org/10.1016/j.jamcollsurg.2012.06.417
Funding
This work was funded by the National Health and Medical Research Council (NHMRC), the Victorian Government’s Operational Infrastructure Support Program, the Swiss National Science Foundation (310030, 146923), Matching Funds at the University of Zurich and the Zurich Heart House, Zurich, Switzerland. PD was supported by the German Research Foundation. JS was supported by the German Society of Cardiology. KP is a Principal Research Fellow of the NHMRC.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Diehl, P., Gaul, D.S., Sogl, J. et al. The effect of oxygen in Sirt3-mediated myocardial protection: a proof-of-concept study in cultured cardiomyoblasts. J Thromb Thrombolysis 46, 102–112 (2018). https://doi.org/10.1007/s11239-018-1677-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-018-1677-3