Abstract
Carotid intima-media thickness (CIMT) is a good surrogate for atherosclerosis. Hyperhomocysteinemia is an independent risk factor for cardiovascular diseases. We aim to investigate the relationships between homocysteine (Hcy) related biochemical indexes and CIMT, the associations between Hcy related SNPs and CIMT, as well as the potential gene–gene interactions. The present study recruited full siblings (186 eligible families with 424 individuals) with no history of cardiovascular events from a rural area of Beijing. We examined CIMT, intima-media thickness for common carotid artery (CCA-IMT) and carotid bifurcation, tested plasma levels for Hcy, vitamin B6 (VB6), vitamin B12 (VB12) and folic acid (FA), and genotyped 9 SNPs on MTHFR, MTR, MTRR, BHMT, SHMT1, CBS genes. Associations between SNPs and biochemical indexes and CIMT indexes were analyzed using family-based association test analysis. We used multi-level mixed-effects regression model to verify SNP-CIMT associations and to explore the potential gene–gene interactions. VB6, VB12 and FA were negatively correlated with CIMT indexes (p < 0.05). rs2851391 T allele was associated with decreased plasma VB12 levels (p = 0.036). In FABT, CBS rs2851391 was significantly associated with CCA-IMT (p = 0.021) and CIMT (p = 0.019). In multi-level mixed-effects regression model, CBS rs2851391 was positively significantly associated with CCA-IMT (Coef = 0.032, se = 0.009, raw p < 0.001) after Bonferoni correction (corrected α = 0.0056). Gene–gene interactions were found between CBS rs2851391 and BHMT rs10037045 for CCA-IMT (p = 0.011), as well as between CBS rs2851391 and MTR rs1805087 for CCA-IMT (p = 0.007) and CIMT (p = 0.022). Significant associations are found between Hcy metabolism related genetic polymorphisms, biochemical indexes and CIMT indexes. There are complex interactions between genetic polymorphisms for CCA-IMT and CIMT.
Similar content being viewed by others
Introduction
Carotid intima-media thickness (CIMT), a technique to monitor carotid artery wall alterations using ultrasonography, is a noninvasive measurement of preclinical atherosclerosis [1]. It has often been considered as a surrogate of early atherosclerosis and is widely used for cardiovascular risk stratification [2].
Hyperhomocysteinemia is an independent risk factor for arteriosclerotic vascular diseases [3, 4]. However, the association between elevated plasma homocysteine (Hcy) and CIMT remains controversial [5]. This prompts the need for genetic association studies to investigate the involvement of Hcy in early stage of atherogenesis.
Hcy can be influenced by genetic factors. Altered functioning of catalytic enzymes in Hcy metabolic pathways caused by gene mutations may lead to inhibition of certain pathways and elevation of plasma Hcy level [6, 7]. Methylenetetrahydrofolate reductase (MTHFR), methionine synthase (MTR), methionines synthase reductase (MTRR), betaine-homocysteine methyltransferase (BHMT) are enzymes in Hcy remethylation pathway. Cystathionine-β-synthase (CBS) is the key enzyme in Hcy transsulfuration pathway. Serine hydroxymethyltransferase 1 (SHMT1) sequesters 5-methyltetrahydrofolate, a co-substrate for homocysteine remethylation, thus to inhibit Hcy metabolism through methionine synthesis pathway. Genetic association studies between encoding genes of these enzymes and cardiovascular studies are previously studied [8, 9].
Different enzymes in Hcy metabolic pathways interact with each other. Complex interactions between variations of corresponding genes, especially interactions between functional polymorphisms, can contribute to Hcy metabolic disorder and subsequent diseases. Interactions between MTHFR 677 C>T (rs1801133) and CBS 844ins68 [10–12], MTHFR 677 C>T and MTRR 66 A>G (rs1801394) [13] were found with regard to Hcy. Same interactions were shown to elevate neural tube defects risk [14]. However, the interactive effects of Hcy related SNPs on CIMT were not fully explored.
To investigate the associations between Hcy metabolism related SNPs, Hcy related biochemical indexes and CIMT, we conducted a sib pair study in Chinese population and genotyped 9 cardiovascular diseases associated SNPs on 6 Hcy metabolism related genes using candidate gene approach. The aims of the present study are: (1) to explore associations between SNPs and Hcy, vitamin B6 (VB6), vitamin B12 (VB12), folic acid (FA) levels; (2) to test the associations between SNPs and CIMT indexes; (3) to explore the potential gene–gene interactions for CIMT indexes.
Methods
Study design
The current study nested in Fangshan Family-based Ischemic Stroke Study In China (FISSIC) program. FISSIC has been described in details elsewhere [15]. In brief, it is an ongoing family-based genetic epidemiological study starting from June 2005. We recruited Northern Chinese Han pedigrees from communities in Fangshan District, Beijing, China. The inclusion criteria for this study were: (1) >40 years old; (2) full siblings; (3) with no medical history of cardiovascular events (including angina pectoris, myocardial infarction, sudden cardiac death, ischemic stroke, hemorrhagic stroke, transient ischemic attack, or other cardiovascular diseases) or antihyperlipidemia treatment at base line. The exclusion criteria were: (1) receiving extra vitamin B or folic acid supplements regularly; (2) receiving drugs which can influence Hcy levels (methotrexated, isoniazide, azathioprine, thiazide diuretics); (3) incomplete genotyping data or other important parameters.
The present study included 186 eligible families with 424 individuals. Written informed consent was obtained from every participant. This project was approved by the Ethics Committee of Peking University Health Science Center, Beijing, China.
Carotid intima-media thickness measurement
Every participant was scanned by one of the two trained ultrasonographers with high-resolution B-mode ultrasound system (Acuson Inc., Mountain View, CA, USA), using 7.5- to 10.0-MHz linear transducers according to study protocol.
Bilateral carotid arteries were scanned from its origin to bifurcation in transverse plane through an anterolateral approach. Longitudinal views were obtained in three segments: (1) distal common carotid artery (CCA) (2 cm proximal to the dilatation of the carotid bulb); (2) proximal CCA (1 cm proximal to the dilatation of the carotid bulb); (3) bifurcation (1 cm proximal to the flow divider). The B-mode ultrasound images were recorded for at least 6 cardiac cycles when the intimal–lumen interface of far wall was displayed as a sharp-edged continuous straight line.
IMT is a double-line pattern represented as the area between the artery luminal edge and media-adventitia interface [1]. IMT was analyzed by 3 qualified readers blinded to participants’ information using semi-automatic edge-detection software (Vasular Research Tool Carotid Analyzer, Medical Imaging Applications, Coralville, IA) in regions free of plaques. The mean IMT of far wall was recorded and was involved in further calculation. In each segment, we measured IMT both in diastole and systole frames and averaged the two values. The common carotid artery IMT (CCA-IMT) was the mean of 4 values obtained in bilateral distal and proximal CCA segments. The bifurcation IMT (Bif-IMT) was the mean of values in bilateral bifurcation segments. Carotid IMT (CIMT) was the mean of IMT in all segments.
To control the quality of IMT measurement, the reliability of scanning and reading procedures was evaluated. The intra-class correlation coefficients for two sonographers were both over 0.93, while the inter-class correlation coefficient was 0.90. The intra-reader correlation coefficients were 0.95 and 0.90 for CCA-IMT and Bif-IMT. The inter-reader correlation coefficients were 0.88 and 0.86 for CCA-IMT and Bif-IMT.
Anthropometric measurements and biochemical analyses
The details of anthropometric measurements [including height (m), weight (kg), body mass index (BMI, kg/m2), systolic blood pressures (SBP, mmHg), diastolic blood pressures (DBP, mmHg), smoking status, drinking status] and biochemical analyses [fasting blood glucose (FBG, mmol/L), total cholesterol (TC, mmol/L), total triglycerides (TG, mmol/L), high density lipoprotein cholesterol (HDL-C, mmol/L), low density lipoprotein cholesterol (LDL-C, mmol/L)] were described elsewhere [15]. Medical history of cardiovascular events was collected through local surveillance system consisting of community medical centers, township hospitals and district hospitals. Current and ex-smokers were both treated as smokers during the analysis, as well as drinkers. Hypertension was defined as a diagnosis of hypertension, antihypertensive therapy, SBP ≥ 140 mmHg or DBP ≥ 90 mmHg during examination. Diabetes was defined as a diagnosis of diabetes, antidiabetic therapy, or FBG ≥ 7.0 mmol/L.
Assessment of homocysteine, vitamin B6, vitamin B12 and folic acid levels
We collected fasting venous blood sample from every participant. Hcy (μmol/L) (Mindray, Shenzhen, China), VB6 (pg/ml), VB12 (pg/ml), FA (pg/ml) (Cusabio, Wuhan, China) and plasminogen activator inhibitor-1 (PAI-1, pg/ml) (Boster, Wuhan, China) concentrations were assessed by commercially available ELISA kits using TECAN GENios Plus enzyme-linked immunosorbent assay reader (TECAN, Grödig, Austria).
SNPs selection and genetic analysis
We investigated 9 single nucleotide polymorphisms (SNPs) in 6 well-studied candidate genes (MTHFR, MTR, MTRR, BHMT, SHMT1, CBS) involved in homocysteine metabolism. SNPs previously reported to be associated with homocysteine levels or cardiac-cerebral vascular diseases were selected.
DNA was extracted from blood samples. We performed DNA genotyping using MassARRAY iPLEX platform (Sequenom Inc, San Diego, California, USA) following the manufacturer’s protocol. We assessed SNP genotypes by MassARRAY Typer Analyzer version 4.0. The call rates for 9 SNPs were all above 99.0%. To verify reproducibility, a randomly chosen subgroup of 5% samples went through repeat analysis. The results of these duplicated samples were 100% consistent.
Statistical analysis
We analyzed TG, Hcy, VB6, VB12, FA, PAI-1 data on natural logarithmic scale owing to their skewed distribution. Continuous variables were described as the mean ± standard deviation and Student’s t test was adopted to compare means across gender groups. Categorical variables were described as frequency and proportion, and Pearson’s χ2 test was used for comparisons between gender groups.
We investigated correlations between Hcy related biochemical indexes and CIMT indexes, and the mean levels of these biochemical indexes in every SNP genotype groups.
We estimated Hardy–Weinberg equilibrium (HWE) for SNPs in all participants and found no violation (Table 4). When investigated under additive genetic models, genotypes with 0, 1 or 2 risk allele copies were coded as 0, 1 and 2 respectively. When investigated under dominant genetic models, genotypes with 0 risk allele copies were coded as 0, while genotypes with 1 or 2 risk allele copies were coded as 1.
Family-based association test in genetic analyses (FBAT) is a method testing for linkage as well as association using familial data. It is built on the original transmission disequilibrium test (TDT) method and is suitable for extended pedigrees such as sib pairs [16]. We used FBAT to estimate associations between SNPs and Hcy related biochemical indexes, as well as CIMT indexes under additive models.
To replicate the significant findings in FBAT, we employed multi-level mixed-effects regression model, which can accommodate correlation between full siblings. Bonferoni-corrected α value was used as the level of significance. SNPs with p < 0.05/9 = 0.0056 were considered significant.
We estimated gene–gene interactions between SNPs significantly associated with CIMT indexes and other SNPs under dominant model by adding multiplicative terms in multi-level mixed-effects regression models.
FBAT analysis was conducted using PBAT software (v3.6). Other statistical analyses were performed by STATA (version 13, Stata Corporation, Texas, USA).
Results
Basic characteristics of participants and sib pairs
The correlations between CCA-IMT and Bif-IMT were 0.754 and 0.786 in men and women respectively. Compared with males, females had lower rates of smoking and drinking. Females also had lower SBP and DBP levels, as well as higher HDL-C levels. The CIMT indexes for females were thinner. The prevalence of hypertension in females was lower than in males (Table 1).
Correlations between biochemical indexes and carotid intima-media thickness
VB6 (CCA-IMT: Corr = −0.283, p < 0.001; Bif-IMT: Corr = −0.234, p < 0.001; CIMT: Corr = −0.277, p < 0.001), VB12 (CCA-IMT: Corr = −0.194, p = 0.002; Bif-IMT: Corr = −0.173, p = 0.007; CIMT: Corr = −0.198, p = 0.002) and FA (CCA-IMT: Corr = −0.371, p < 0.001; Bif-IMT: Corr = −0.280, p < 0.001; CIMT: Corr = −0.363, p < 0.001); were negatively correlated with CIMT indexes. No correlations were found between Hcy, PAI-1 and CIMT indexes (Table 2).
Associations between homocysteine metabolism related SNPs and biochemical indexes
rs1801133 T allele was associated with elevated plasma Hcy levels (p = 0.012). rs1532268 A allele was associated with elevated plasma VB6 levels (p = 0.010). rs2851391 T allele was associated with decreased plasma VB12 levels (p = 0.036) (Table 3).
Associations between homocysteine metabolism related SNPs and carotid intima-media thickness in family-based association test analysis
When analyzing associations between 9 SNPs and CIMT indexes using family-based association test analysis (FBAT), we found that CBS rs2851391 was significantly associated with CCA-IMT (p = 0.021) and CIMT (p = 0.019), MTRR rs1532268 was significantly associated with CCA-IMT (p = 0.040). No significant association was found between Bif-IMT and homocysteine metabolism related SNPs (Table 4).
Associations between homocysteine metabolism related SNPs and carotid intima-media thickness in multi-level mixed-effects regression model
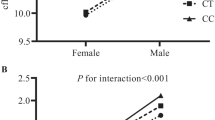
To replicate FBAT result, we further analyzed associations between 9 SNPs and CIMT indexes using multi-level mixed-effects regression model. SHMT1 rs11868708 was associated with CCA-IMT (Coef = 0.007, se = 0.008, raw p = 0.013) and CIMT (Coef = 0.011, se = 0.005, raw p = 0.024). CBS rs2851391was associated with CCA-IMT (Coef = 0.032, se = 0.009, raw p < 0.001), Bif-IMT (Coef = 0.025, se = 0.011, raw p = 0.019) and CIMT (Coef = 0.023, se = 0.010, raw p = 0.018).
After Bonferoni correction, CBS rs2851391 remained significantly associated with CCA-IMT (α = 0.0056). Compared with individuals with no T alleles on rs2851391, every extra T allele can result in 0.032 millimeters (mm) thickening of CIMT. No associations between SNPs and Bif-IMT or CIMT reached Bonferoni corrected α threshold (Table 5).
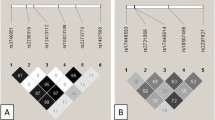
Gene–gene interactions between CBS rs2851391 and other homocysteine related SNPs on carotid intima-media thickness
Gene–gene interactive effects were analyzed under dominant genetic model in multi-level mixed-effects regression model. An interaction was found between CBS rs2851391 and BHMT rs10037045 for CCA-IMT (p = 0.011). The combination of CBS rs2851391 CT/TT and BHMT rs10037045 AT/TT genotypes can increase CCA-IMT by 0.080 mm. Interactions were found between CBS rs2851391 and MTR rs1805087 for CCA-IMT (p = 0.007) and CIMT (p = 0.022). The combination of CBS rs2851391 CT/TT and MTR rs1805087 AG/GG genotypes can increase CCA-IMT by 0.101 mm and CIMT by 0.089 mm (Table 6).
Discussion
The accessibility and reproducibility of CIMT measurement varies significantly at different carotid artery segments [over 90% for CCA, 60–80% for bifurcation and 30–50% for internal carotid artery (ICA)] [17]. In the present study, we recorded images of bilateral ICA. However, ICA IMT can only be clearly measured in 38.6% participants. Thus it was not involved in further statistical analysis. The associations between CIMT at various segments and cardiovascular diseases are also different. Thickening of CCA-IMT may reflect the pathological changes of medial hypertrophy as a result of smooth muscle cell hyperplasia or fibrocellular hypertrophy rather than the formation of atherosclerotic plaque [18]. Epidemiological studies suggested CCA-IMT as a strong predictor for cardiovascular events. It was associated with myocardial infarction and stroke [2, 19], which was later proved by a meta-analysis [20]. Carotid bifurcation is a region where blood in carotid artery flows into two vessels of unequal sizes. The consequent sudden changes of hemodynamic factors, such as variations in flow velocity, shear stress and turbulence, make carotid bifurcation an atherosclerosis-prone location [21, 22]. Ebrahim et al. reported that the associations between CCA-IMT and stroke were independent of carotid plaques, whereas associations between Bif-IMT and ischemic heart disease can be largely explained by the presence of carotid plaques [23].
B-group vitamins are essential coenzymes in Hcy metabolism. Evidences indicated that increased CIMT was associated with higher Hcy and lower folic acid levels [24]. Although we observed no associations between Hcy levels and CIMT indexes, significant negative associations between FA, VB6, VB12 and CIMT indexes were found in the present study. B vitamins (FA, VB6, VB12) can prevent atherogenesis through their antioxidant properties and their ability to lower plasma homocysteine levels [25, 26]. However, the effect of folic acid and vitamin B supplementation on CIMT progression was still controversial [27].
Plasminogen activator inhibitor-1, a component of the plasminogen/plasmin system, was considered as a link between inflammation, insulin resistance and vascular risks, such as atherogenesis and atherothrombosis [28, 29]. PAI-1 was suggested to be a predictor for cardiovascular disease [30]. However, associations between PAI-1 and CIMT were not consistent in different studies [31, 32]. We tested PAI-1 in the present study but found no associations between PAI-1 and CIMT indexes.
MTHFR 677 C>T (rs1801133) is the most frequently studied functional genetic polymorphism proved to be independently associated with folate, homocysteine, and vascular endothelial dysfunction [33–35]. However, association between MTHFR 677 C>T genotype and CIMT in different ethnic populations remains controversial [5, 36–38]. There is also no evidence that MTHFR 677 C>T has an effect on coronary artery disease [39]. In the present study, we observed an association between rs1801133 T allele and elevated plasma Hcy levels, but found null association between MTHFR 677 C>T and CIMT indexes. These results indicated that the thickening of CIMT might be a pathological process resulting from complex metabolic disorders rather than isolated hyperhomocysteinemia.
SHMT1 rs11868708 risk allele conferred a 1.46-fold risk of ischemic stroke in Singaporean Chinese. The total number of risk alleles on MTRR rs16879248, SHMT1 rs11868708, TCN2 rs1173570 showed a cumulative effect on ischemic stroke [40]. These three SNPs were genotyped in the present study. The association between SHMT1 rs11868708 and CCA-IMT (raw p = 0.013) didn’t reach Bonferoni corrected α threshold. More studies are needed to investigate the role SHMT1 rs11868708 plays in atherogenesis and subsequent cardiovascular diseases.
Cystathionine-β-synthase (CBS) transforms homocysteine to cystathionine in the assistance of vitamin B6. CBS 68-bp insertion (844ins68) carriers have been reported to lower plasma Hcy levels [41]. Rs2851391 located in the intron of CBS. A GWAS meta-analysis showed that CBS rs234709 and CBS rs2851391 were associated with Hcy concentrations [9]. Zinck et al. discussed that rs2851391 variants might reduce the activity of CBS, and thus was positively associated with homocysteine levels [42]. Rs2851391 genetic polymorphism is associated with neural tube defects. Its risk genotype has a 2.0-fold risk of spina bifida [43]. Hsu et al. indicated that rs2851391 polymorphisms were associated with changes in postmethionine load Hcy levels, but it was not associated with recurrent stroke risk [44]. In the present study, we identified CBS rs2851391 as a risk factor for CCA-IMT but not Bif-IMT. This might result from the different pathology for CCA-IMT and Bif-IMT thickening. CBS rs2851391 T allele showed a marginal association with decreased plasma VB12 level, which might be a possible explanation for its association with CCA-IMT.
Methionine synthase (MTR) catalyzes the remethylation of homocysteine to methionine [45]. MTR rs1805087 (A2756G) is a well-studied genetic polymorphism. Laraqui et al. defined Hcy level ≥15 μmol/l as hyperhomocyteinemia (HHcy), and found that MTR rs1805087 (A2756G) G allele contributed a 2.0-fold risk for HHcy [46]. The G allele can also increase DNA methylation level [47]. Masud et al. reported significant associations of rs1805087 with coronary artery disease under additive and dominant models [48].
Gene–gene interactions between homocysteine related SNPs are studied before. Many studies focus on interactions between MTR rs1805087 and genetic polymorphisms of other homocysteine metabolism related genes. The combination of MTR A2756G AG/GG, MTHFR 677 CT/TT and MTHFR 1298 AC/AA genotypes contributes significantly to extremely high Hcy plasma levels [49]. Interaction between MTR A2756G and MTRR A66G may lead to an increase of infants’ neural tube defects risks [50]. An existence of gene–gene interaction between rs1801133, rs662 and rs1805087 was reported for coronary artery disease [48]. MTR rs1805087 is located in the AdoMet-binding region, this polymorphism results in a nonconservative substitution of aspartic acid residue by glycine residue, which could influence the secondary structure and function of the protein [51]. In the present study, we observed an association of distinct CBS rs2851391/MTR rs1805087 genotype combination with elevated CCA-IMT and CIMT, which might be an indication of interaction between Hcy remethylation pathway and transsulfuration pathway. BHMT rs10037045 is reported to be a risk factor for ischemic stroke [52]. This SNP is not well studied before. More researches are needed to understand the role BHMT rs10037045 plays in atherogenesis and cardiovascular diseases, and its interaction with CBS rs2851391.
Compared with parent-offspring trios study, sib pair study is more suitable for late-onset diseases, since the parents’ phenotypic and genotypic traits may not be available at the time offspring develops the certain disease. What’s more, sib pairs are more closely matched for age and environmental factors than other relative pairs [53]. The shared genetic background for siblings from same pedigree prevented the problem of population stratification, a cause for false positive associations in population based case-control studies. As a consequent, the power of sib pair study is relatively low [54].
The present study has some limitations. Firstly, we only selected 9 SNPs located on homocysteine related genes using candidate gene approach. These 9 SNPs are previously reported to be associated with Hcy levels or cardiovascular diseases, or to have interactions with risk SNPs for cardiovascular diseases. Thus, the aim of the present family-based sib pair study is to verify positive results found in population based studies, rather than to identify new risk SNPs. Secondly, the present study is a cross-sectional study. The causality of the associations is thus compromised. However, since human genetic profile is unchangeable during life time, the genetic risk factors can be considered as being prior to disease occurrence. On the other hand, this can’t deny the need for longitudinal studies to elucidate the relationships between homocysteine related SNPs and CIMT or CIMT changes. Thirdly, CIMT, especially CCA-IMT, is a good surrogate for cardiovascular events. The associations between risk SNPs and CIMT indexes suggest a possible influence of these SNPs on cardiovascular diseases. However, it is of greater value to directly elaborate the associations between SNPs and individual diseases. FISSIC study is an ongoing family-based genetic epidemiological study. Our next goal is to investigate the role genetic risk factors play in ischemic stroke, coronary artery disease and type 2 diabetes.
Conclusions
Significant associations are found between Hcy related genetic polymorphisms, biochemical indexes and carotid intema-media thickness. There are complex interactions between Hcy related genetic polymorphisms.
References
Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R (1986) Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation 74(6):1399–1406
O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr (1999) Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 340(1):14–22. doi:10.1056/nejm199901073400103
Boushey CJ, Beresford SA, Omenn GS, Motulsky AG (1995) A quantitative assessment of plasma homocysteine as a risk factor for vascular disease: probable benefits of increasing folic acid intakes. Jama 274(13):1049–1057
Graham IM, Daly LE, Refsum HM, Robinson K, Brattström LE, Ueland PM, Palma-Reis RJ, Boers GH, Sheahan RG, Israelsson B (1997) Plasma homocysteine as a risk factor for vascular disease: the European Concerted Action Project. Jama 277(22):1775–1781
Durga J, Verhoef P, Bots ML, Schouten E (2004) Homocysteine and carotid intima-media thickness: a critical appraisal of the evidence. Atherosclerosis 176(1):1–19
Refsum M H, Ueland M PM, Nygård M O, Vollset M, Dr. PH, SE (1998) Homocysteine and cardiovascular disease. Annu Rev Med 49(1):31–62
Miyaki K (2010) Genetic polymorphisms in homocysteine metabolism and response to folate intake: a comprehensive strategy to elucidate useful genetic information. Journal of epidemiology 20(4):266–270
Williams SR, Yang Q, Chen F, Liu X, Keene KL, Jacques P, Chen W-M, Weinstein G, Hsu F-C, Beiser A (2014) Genome-wide meta-analysis of homocysteine and methionine metabolism identifies five one carbon metabolism loci and a novel association of ALDH1L1 with ischemic stroke. PLoS Genet 10:e1004214
van Meurs JB, Pare G, Schwartz SM, Hazra A, Tanaka T, Vermeulen SH, Cotlarciuc I, Yuan X, Mälarstig A, Bandinelli S (2013) Common genetic loci influencing plasma homocysteine concentrations and their effect on risk of coronary artery disease. Am J Clin Nutr 98(3):668–676
Nienaber-Rousseau C, Ellis SM, Moss SJ, Melse-Boonstra A, Towers GW (2013) Gene–environment and gene–gene interactions of specific MTHFR, MTR and CBS gene variants in relation to homocysteine in black South Africans. Gene 530(1):113–118
STEFANO V, Dekou V, Nicaud V, Chasse J, London J, Stansbie D, Humphries S, Gudnason V (1998) Linkage disequilibrium at the cystathionine β synthase (CBS) locus and the association between genetic variation at the CBS locus and plasma levels of homocysteine. Ann Hum Genet 62(6):481–490
Dekou V, Gudnason V, Hawe E, Miller GJ, Stansbie D, Humphries SE (2001) Gene-environment and gene-gene interaction in the determination of plasma homocysteine levels in healthy middle-aged men. Thromb Haemost 85(1):67–74
Yang Q-H, Botto LD, Gallagher M, Friedman J, Sanders CL, Koontz D, Nikolova S, Erickson JD, Steinberg K (2008) Prevalence and effects of gene–gene and gene–nutrient interactions on serum folate and serum total homocysteine concentrations in the United States: findings from the third National Health and Nutrition Examination Survey DNA Bank. Am J Clin Nutr 88(1):232–246
Relton C, Wilding C, Pearce M, Laffling A, Jonas P, Lynch S, Tawn E, Burn J (2004) Gene–gene interaction in folate-related genes and risk of neural tube defects in a UK population. J Med Genet 41(4):256–260
Tang X, Hu Y, Chen D, Zhan S, Zhang Z, Dou H (2007) The Fangshan/family-based ischemic stroke study in China (FISSIC) protocol. BMC Med Genet 8:60. doi:10.1186/1471-2350-8-60
Laird NM, Horvath S, Xu X (2000) Implementing a unified approach to family-based tests of association. Genet Epidemiol 19(S1):S36–S42
Roman MJ, Naqvi TZ, Gardin JM, Gerhard-Herman M, Jaff M, Mohler E (2006) Clinical application of noninvasive vascular ultrasound in cardiovascular risk stratification: a report from the American Society of Echocardiography and the Society of Vascular Medicine and Biology. J Am Soc Echocardiogr 19(8):943–954. doi:10.1016/j.echo.2006.04.020
Touboul PJ, Hennerici MG, Meairs S, Adams H, Amarenco P, Bornstein N, Csiba L, Desvarieux M, Ebrahim S, Hernandez Hernandez R, Jaff M, Kownator S, Naqvi T, Prati P, Rundek T, Sitzer M, Schminke U, Tardif JC, Taylor A, Vicaut E, Woo KS (2012) Mannheim carotid intima-media thickness and plaque consensus (2004–2006–2011). An update on behalf of the advisory board of the 3rd, 4th and 5th watching the risk symposia, at the 13th, 15th and 20th European Stroke Conferences, Mannheim, Germany, 2004, Brussels, Belgium, 2006, and Hamburg, Germany, 2011. Cerebrovasc Dis (Basel, Switzerland) 34(4):290–296. doi:10.1159/000343145
Bots ML, Hoes AW, Koudstaal PJ, Hofman A, Grobbee DE (1997) Common carotid intima-media thickness and risk of stroke and myocardial infarction: the Rotterdam Study. Circulation 96(5):1432–1437
Lorenz MW, Markus HS, Bots ML, Rosvall M, Sitzer M (2007) Prediction of clinical cardiovascular events with carotid intima-media thickness: a systematic review and meta-analysis. Circulation 115(4):459–467. doi:10.1161/circulationaha.106.628875
Zarins CK, Giddens DP, Bharadvaj BK, Sottiurai VS, Mabon RF, Glagov S (1983) Carotid bifurcation atherosclerosis. Quantitative correlation of plaque localization with flow velocity profiles and wall shear stress. Circ Res 53(4):502–514
Ku DN, Giddens DP, Zarins CK, Glagov S (1985) Pulsatile flow and atherosclerosis in the human carotid bifurcation. Positive correlation between plaque location and low oscillating shear stress. Arteriosclerosis (Dallas, Tex) 5(3):293–302
Ebrahim S, Papacosta O, Whincup P, Wannamethee G, Walker M, Nicolaides AN, Dhanjil S, Griffin M, Belcaro G, Rumley A, Lowe GD (1999) Carotid plaque, intima media thickness, cardiovascular risk factors, and prevalent cardiovascular disease in men and women: the British Regional Heart Study. Stroke 30(4):841–850
Kalra L, Iveson E, Rambaran C, Sherwood R, Chowienczyk P, Ritter J, Shah A, Forrester T (2008) Homocysteine, migration and early vascular impairment in people of African descent. Heart 94(9):1171–1174. doi:10.1136/hrt.2007.132670
Shirodaria C, Antoniades C, Lee J, Jackson CE, Robson MD, Francis JM, Moat SJ, Ratnatunga C, Pillai R, Refsum H (2007) Global improvement of vascular function and redox state with low-dose folic acid implications for folate therapy in patients with coronary artery disease. Circulation 115(17):2262–2270
Yilmaz H, Sahin S, Sayar N, Tangurek B, Yilmaz M, Nurkalem Z, Onturk E, Cakmak N, Bolca O (2007) Effects of folic acid and N-acetylcysteine on plasma homocysteine levels and endothelial function in patients with coronary artery disease. Acta Cardiol 62(6):579–585
Debreceni B, Debreceni L (2014) The role of homocysteine-lowering B-vitamins in the primary prevention of cardiovascular disease. Cardiovasc Ther 32(3):130–138
Juhan-Vague I, Alessi M, Vague P (1991) Increased plasma plasminogen activator inhibitor 1 levels. A possible link between insulin resistance and atherothrombosis. Diabetologia 34(7):457–462
Fortenberry YM (2013) Plasminogen activator inhibitor-1 inhibitors: a patent review (2006–present). Expert Opin Ther Patents 23(7):801–815
Tofler G, Massaro J, O’Donnell C, Wilson P, Vasan R, Sutherland P, Meigs J, Levy D, D’Agostino R (2016) Plasminogen activator inhibitor and the risk of cardiovascular disease: the Framingham Heart Study. Thromb Res 140:30–35
Adly AAM, Elbarbary NS, Ismail EAR, Hassan SR (2014) Plasminogen activator inhibitor-1 (PAI-1) in children and adolescents with type 1 diabetes mellitus: relation to diabetic micro-vascular complications and carotid intima media thickness. J Diabetes Complic 28(3):340–347
Karasek D, Vaverkova H, Halenka M, Jackuliakova D, Frysak Z, Slavik L, Novotny D (2011) Prothrombotic markers in asymptomatic dyslipidemic subjects. J Thromb Thrombolysis 31(1):27–36. doi:10.1007/s11239-010-0474-4
Antoniades C, Shirodaria C, Leeson P, Baarholm OA, Van-Assche T, Cunnington C, Pillai R, Ratnatunga C, Tousoulis D, Stefanadis C (2009) MTHFR 677 C > T Polymorphism reveals functional importance for 5-methyltetrahydrofolate, not homocysteine, in regulation of vascular redox state and endothelial function in human atherosclerosis. Circulation 119(18):2507–2515
Imamura A, Okumura K, Matsui H, Mizuno T, Ogawa Y, Imai H, Numaguchi Y, Sakai K, Murohara T (2004) Endothelial nitric oxide synthase and methylenetetrahydrofolate reductase gene polymorphisms are associated with endothelial dysfunction in young, healthy men. Can J Cardiol 20(12):1229–1234
Lin Y, Rui X, Guohua L, Yucai Y, Jiamin L, Suhua Y (2014) GW25-e3364 MTHFR C677T gene mutation affect the level of plasma homocysteine but do not related to early renal damage in hypertensive patients. J Am Coll Cardiol 64 (16_S)
Kelemen LE, Anand SS, Hegele RA, Stampfer MJ, Rosner B, Willett WC, Montague PA, Lonn E, Vuksan V, Teo KK (2004) Associations of plasma homocysteine and the methylenetetrahydrofolate reductase C677T polymorphism with carotid intima media thickness among South Asian, Chinese and European Canadians. Atherosclerosis 176(2):361–370
Liu C, Chen C, Chiang H, Kuo C, Huang C, Cheng W, Wei Y-H, Chen H (2007) B-group vitamins, MTHFR C677T polymorphism and carotid intima-media thickness in clinically healthy subjects. Eur J Clin Nutr 61(8):996–1003
Panayiotou A, Nicolaides A, Griffin M, Tyllis T, Georgiou N, Martin RM, Bond D, Tziakouri-Shiakalli C, Fessas C, Deltas C (2009) Serum total homocysteine, folate, 5,10-methylenetetrahydrofolate reductase (MTHFR) 677 C–>T genotype and subclinical atherosclerosis. Expert Opin Ther Targets 13(1):1–11. doi:10.1517/14728220802560281
van Meurs JB, Pare G, Schwartz SM, Hazra A, Tanaka T, Vermeulen SH, Cotlarciuc I, Yuan X, Malarstig A, Bandinelli S, Bis JC, Blom H, Brown MJ, Chen C, Chen YD, Clarke RJ, Dehghan A, Erdmann J, Ferrucci L, Hamsten A, Hofman A, Hunter DJ, Goel A, Johnson AD, Kathiresan S, Kampman E, Kiel DP, Kiemeney LA, Chambers JC, Kraft P, Lindemans J, McKnight B, Nelson CP, O’Donnell CJ, Psaty BM, Ridker PM, Rivadeneira F, Rose LM, Seedorf U, Siscovick DS, Schunkert H, Selhub J, Ueland PM, Vollenweider P, Waeber G, Waterworth DM, Watkins H, Witteman JC, den Heijer M, Jacques P, Uitterlinden AG, Kooner JS, Rader DJ, Reilly MP, Mooser V, Chasman DI, Samani NJ, Ahmadi KR (2013) Common genetic loci influencing plasma homocysteine concentrations and their effect on risk of coronary artery disease. Am J Clin Nutr 98(3):668–676. doi:10.3945/ajcn.112.044545
Low H-Q, Chen CP, Kasiman K, Thalamuthu A, Ng S-S, Foo J-N, Chang H-M, Wong M-C, Tai E-S, Liu J (2011) A comprehensive association analysis of homocysteine metabolic pathway genes in Singaporean Chinese with ischemic stroke. PloS One 6(9): e24757
Tsai MY, Yang F, Bignell M, Aras O, Hanson NQ (1999) Relation between plasma homocysteine concentration, the 844ins68 variant of the cystathionine beta-synthase gene, and pyridoxal-5′-phosphate concentration. Mol Genet Metab 67(4):352–356. doi:10.1006/mgme.1999.2874
Zinck JW, de Groh M, MacFarlane AJ (2015) Genetic modifiers of folate, vitamin B-12, and homocysteine status in a cross-sectional study of the Canadian population. Am J Clin Nutr 101(6):1295–1304. doi:10.3945/ajcn.115.107219
Shaw GM, Lu W, Zhu H, Yang W, Briggs FB, Carmichael SL, Barcellos LF, Lammer EJ, Finnell RH (2009) 118 SNPs of folate-related genes and risks of spina bifida and conotruncal heart defects. BMC Med Genet 10:49. doi:10.1186/1471-2350-10-49
Hsu F-C, Sides E, Mychaleckyj J, Worrall B, Elias G, Liu Y, Chen W-M, Coull B, Toole J, Rich S (2011) Transcobalamin 2 variant associated with poststroke homocysteine modifies recurrent stroke risk. Neurology 77(16):1543–1550
Blom HJ, Smulders Y (2011) Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J Inherit Metab Dis 34(1):75–81. doi:10.1007/s10545-010-9177-4
Laraqui A, Allami A, Carrie A, Raisonnier A, Coiffard AS, Benkouka F, Bendriss A, Benjouad A, Bennouar N, El Kadiri N, Benomar A, Fellat S, Benomar M (2007) Relation between plasma homocysteine, gene polymorphisms of homocysteine metabolism-related enzymes, and angiographically proven coronary artery disease. Eur J Intern Med 18(6):474–483. doi:10.1016/j.ejim.2007.02.020
Weiner AS, Boyarskikh UA, Voronina EN, Mishukova OV, Filipenko ML (2014) Methylenetetrahydrofolate reductase C677T and methionine synthase A2756G polymorphisms influence on leukocyte genomic DNA methylation level. Gene 533(1):168–172. doi:10.1016/j.gene.2013.09.098
Masud R, Qureshi IZ (2011) Tetra primer ARMS-PCR relates folate/homocysteine pathway genes and ACE gene polymorphism with coronary artery disease. Mol Cell Biochem 355(1–2):289–297. doi:10.1007/s11010-011-0866-6
Feix A, Fritsche-Polanz R, Kletzmayr J, Vychytil A, Horl WH, Sunder-Plassmann G, Fodinger M (2001) Increased prevalence of combined MTR and MTHFR genotypes among individuals with severely elevated total homocysteine plasma levels. Am J Kidney Dis 38(5):956–964. doi:10.1053/ajkd.2001.28581
Zhu H, Wicker NJ, Shaw GM, Lammer EJ, Hendricks K, Suarez L, Canfield M, Finnell RH (2003) Homocysteine remethylation enzyme polymorphisms and increased risks for neural tube defects. Mol Genet Metab 78(3):216–221
van der Put NM, van der Molen EF, Kluijtmans LA, Heil SG, Trijbels JM, Eskes TK, Van Oppenraaij-Emmerzaal D, Banerjee R, Blom HJ (1997) Sequence analysis of the coding region of human methionine synthase: relevance to hyperhomocysteinaemia in neural-tube defects and vascular disease. QJM 90(8):511–517
Giusti B, Saracini C, Bolli P, Magi A, Martinelli I, Peyvandi F, Rasura M, Volpe M, Lotta LA, Rubattu S, Mannucci PM, Abbate R (2010) Early-onset ischaemic stroke: analysis of 58 polymorphisms in 17 genes involved in methionine metabolism. Thromb Haemost 104(2):231–242. doi:10.1160/th09-11-0748
Kruglyak L, Lander ES (1995) Complete multipoint sib-pair analysis of qualitative and quantitative traits. Am J Hum Genet 57(2):439–454
Laird NM, Lange C (2006) Family-based designs in the age of large-scale gene-association studies. Nat Rev Genet 7(5):385–394. doi:10.1038/nrg1839
Acknowledgements
The authors gratefully thank for staff of FISSIC study and all the study participants. This study is supported by the Key Project of National Natural Science Foundation of China (81230066) and the National Natural Science Foundation of China (81102177, 81172744, 81473043).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sun, K., Song, J., Liu, K. et al. Associations between homocysteine metabolism related SNPs and carotid intima-media thickness: a Chinese sib pair study. J Thromb Thrombolysis 43, 401–410 (2017). https://doi.org/10.1007/s11239-016-1449-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11239-016-1449-x