Abstract
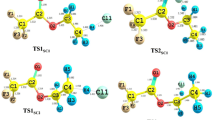
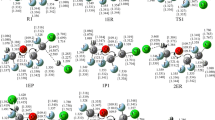
Quantum mechanical calculations are carried out on the reactions of CH3OCHCl2 (DCDME) with Cl atom by means of DFT and couple cluster methods. The geometries of the reactants, products, and transition states involved in the reaction pathways are optimized at BHandHLYP level of theory using 6-311G(d,p) basis set. Transition states are searched on the potential energy surface involved during the reaction channels, and each of the transition states is characterized by the presence of only one imaginary frequency. The existence of transition states on the corresponding potential energy surface is ascertained by performing intrinsic reaction coordinate calculation. Single point energy calculations are performed at CCSD(T) level using the same basis set. The hydrogen abstraction rate constant for the title reaction is calculated at 298 K and atmospheric pressure using the canonical transition state theory including tunneling correction. The calculated value for rate constant as 1.204 × 10−12 cm3 molecule−1 s−1 is found to be in very good agreement with the recent experimental data. The percentage contributions of both reaction channels are also reported at 298 K.



Similar content being viewed by others
References
Atkinson R, Arey J (2003) Chem Rev 103:4605–4638
Placet M, Mann CO, Gilbert RO, Niefer MJ (2000) Atmos Environ 34:2183–2204
Guenther A, Geron C, Pierce T, Lamb B, Harley P, Fall R (2000) Atmos Environ 34:2205–2230
Guenther A, Hewitt CN, Erickson D, Fall R, Geron C, Graedel T, Harley P, Klinger L, Lerdau M, McKay WA, Pierce T, Scholes B, Steinbrecher R, Tallamraju R, Taylor J, Zimmermann P (1995) J Geophys Res 100:8873–8892
Sawyer RF, Harley RA, Cadle SH, Norbeck JM, Slott R, Bravo HA (2000) J Atmos Environ 34:12–14
Wallington TJ, Gushin A, Stein TNN, Platz J, Sehested J, Christensen LK, Nielsen OJ (1998) J Phys Chem A 102:1152–1161
Tsai WT (2005) J Hazard Mater 119:69–78
Sekiya A, Misaki S (2000) J Fluorine Chem 101:215–221
Singh HJ, Mishra BK (2010) J Mol Model 16:1473–1480
Singh HJ, Mishra BK (2011) J Mol Model 17:415–422
Blowers P, Tetrault KF, Morehead YT (2008) Theor Chem Acc 119:369–381
Ravishankara RA, Turnipseed AA, Jensen NR, Barone S, Mills M, Howark CJ, Solomon S (1994) Science 263:71–75
Granier C, Shine KP, Daniel JS, Hansen JE, Lal S, Stordal F (1999) Climate effects of ozone and halocarbon changes in scientific Assessment of Ozone Depletion: 1998, Rep. 44, Global Ozone Research and Monitoring Project, World Meteorological Organization, Geneva, Switzerland
Chandra AK (2012) J Mol Model 18:4239–4247
Zierkiewicz W, Michalska D, Zeegers-Huyskens T (2010) Phys Chem Chem Phys 12:13681–13691
Nishida S, Morita Y, Ueda A, Kobayashi T, Fukui K, Ogasawara K, Sato K, Takui T, Nakasuji K (2008) J Am Chem Soc 130:14954–14955
Dalmasso PR, Taccone Raúl A, Nieto JD, Cometto PM, Lane SI (2010) Atmos Environ 44:1749–1753
Spicer CW, Chapman EG, Finlayson-Pitts BJ, Plastidge RA, Hubbe JM, Fast JD, Berkowitz CM (1998) Nature 394:353–356
Mellouki A, Le Bras G, Sidebottom H (2003) Chem Rev 103:5077–5096
Ezell MJ, Wang W, Ezell AA, Soskin G, Finlayson-Pitts BJ (2002) Phys Chem Chem Phys 4:5813–5820
Finlayson-Pitts BJ, Pitts JN Jr (2000) Chemistry of the upper and lower atmosphere. Academic, New York
Dalmasso PR, Taccone RA, Nietoa JD, PM Comettoa, Lane SI (2008) J Phys Org Chem 21:393–396
Dalmasso PR, Taccone RA, Nietoa JD, Comettoa PM, Teruel MA, Lane SI (2005) Int J Chem Kinet 37:420–426
Truhlar DG, Garrett BC, Klippenstein SJ (1996) J Phys Chem 100:12771–12800
Laidler KJ (2004) Chemical kinetics, 3rd edn. Pearson Education, Delhi
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X,Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr , Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell K, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09, revision B.01. Gaussian Inc., Wallingford
Becke AD (1993) J Chem Phys 98:1372–1377
Pople JA, Head-Gordon M, Raghavachari K (1987) J Chem Phys 87:5968–5975
Alvarez-Idaboy JR, Cruz-Torres A, Galano A, Ruiz-Santoyo ME (2004) J Phys Chem 108:2740–2749
Zhurko G, Zhurko D (2011) ChemCraft 1.6
Gonzalez C, Schlegel HB (1989) J Chem Phys 90:2154–2161
Kuchitsu K structure of free polyatomic molecules basic data (1998) Springer, Berlin 1, 58
Hammond GS (1955) J Am Chem Soc 77:334–338
Truhlar DG, Chuang YY (2000) J Chem Phys 112:1221–1228
Wigner EP (1932) Z Phys Chem B19:203–216
Chase MW Jr, Davies CA, Downey JR Jr, Frurip DJ, McDonald RA, Syverud AN (1985) JANAF thermochemical tables, J Phys Chem Ref Data 14, Suppl 1, 3rd edn
In NIST Chemistry Web Book, NIST Standard Reference Data-base Number 69 (2005) (Vibrational frequency data compiled by T. Shimanouchi). http://www.ccbdb.nist.gov/. Accessed 18 Jan 2013
Acknowledgments
One of the authors, BKM, is thankful to University Grants Commission, New Delhi for providing Dr. D. S. Kothari Post doctoral Fellowship. The financial support in the form of Junior Research Fellowship to DB from CSIR, New Delhi is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mishra, B.K., Chakrabartty, A.K., Bhattacharjee, D. et al. Theoretical study on the kinetics and branching ratios of the gas phase reactions of 1, 1-Dichlorodimethylether (DCDME) with Cl atom. Struct Chem 24, 1621–1626 (2013). https://doi.org/10.1007/s11224-013-0203-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-013-0203-7