Abstract
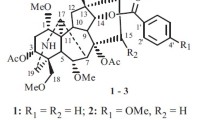
Two alkaloid triterpenoids formulated as C26H35NO3 (1) and C30H45NO2 (2) were isolated from the timer tree Aphanamixis grandifolia. The structure of 1 and 2 was determined by IR, HRESIMS, 1D, and 2D-NMR. Compound 1 was confirmed by X-ray single crystal diffraction. Compound 1 crystallizes in the monoclinic, space group C2 with unit cell parameters a = 53.195(4) Å, b = 7.6339(8) Å, c = 11.202(2) Å, β = 94.6520(2)°. Intermolecular hydrogen bonding and π–π stacking were presented in the molecular packing of 1. The absolute configurations of 1 and 2 were established by comparison of experimental circular dichroism properties with their electronic circular dichroism predicted by molecular modeling DFT calculations.








Similar content being viewed by others
References
Wang XN, Yin S, Fan CQ, Wang FD, Lin LP, Ding J, Yue JM (2006) Org Lett 8:3845–3848
He XF, Wang XN, Gan LS, Yin S, Dong L, Yue JM (2008) Org Lett 10:4327–4330
Luo J, Wang JS, Wang XB, Luo JG, Kong LY (2009) Org Lett 11:2281–2284
Yuan T, Zhu RX, Zhang H, Odecu OA, Yang SP, Liao SG, Yue JM (2010) Org Lett 12:252–255
Nishizawa M, Inoue A, Hayashi Y, Sastrapradja S, Kosela S, Iwashita T (1984) J Org Chem 49:3660–3664
Polonsky J, Varon Z, Arnoux B, Pettit GR, Schmidt JH, Lange LM (1978) J Am Chem Soc 100:2575–2576
Polonsky J, Varon Z, Arnoux B, Pettit GR, Schmidt JH (1978) J Am Chem Soc 100:7731–7733
Zhang XY, Li Y, Wang YY, Cai XH, Feng T, Luo XD (2010) J Nat Prod 73:1385–1388
Sheldrick GM (2008) Acta Cryst A64:112–122
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 09, Revision B.01. Gaussian, Inc., Wallingford
Mora AJ, Delgado G, de Delgado GD, Usubillaga A, Khourib N, Bahsasc A (2001) Acta Cryst c57:638–640
Chen HD, Yang SP, Wu Y, Dong L, Yue JM (2009) J Nat Prod 72:685–689
Sang YS, Zhou CY, Lu AJ, Min ZD, Tan RX (2009) J Nat Prod 72:917–920
Cremer D, Pople JA (1975) J Am Chem Soc 97:1354–1358
Rao ST, Westhof E, Sundaralingam M (1981) Acta Cryst a37:421–425
Etter MC (1990) Acc Chem Res 23:120–126
Bernstein J, Davis RE, Shimoni L, Chang NL (1995) Angew Chem Int Ed 34:1555–1573
Desiraju GR (1995) Angew Chem Int Ed 34:2311–2327
Ren YL, Lantvit DD, de Blanco C, Kardono LBS, Riswan S, Chai H, Cottrell CE, Farnsworth NR, Swanson SM, Ding YQ, Li XC, Marais JPJ, Ferreira D, Kinghorn AD (2010) Tetrahedron 66:5311–5320
Stephens PJ, Harada N (2010) Chirality 22:229–233
Lunazzi L, Mancinelli M, Mazzanti A, Pierini M (2010) J Org Chem 75:5927–5933
McChesney JD, Dou JH, Sindelar RD, Goins DK, Walker LA, Rogers RD (1997) J Chem Crystallogr 27:283–290
Acknowledgment
The research work was partially supported by National Key Scientific and Technological Special Project (Grant No. 2009ZX09502-011).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, XB., Zhang, Y., Wang, JS. et al. Spectroscopic characterizations, X-ray studies, and electronic circular dichroism calculations of two alkaloid triterpenoids. Struct Chem 22, 1241–1248 (2011). https://doi.org/10.1007/s11224-011-9818-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-011-9818-8