Abstract
Oxidation of 1,4-bis(4′-oxo-2′,2′-dimethylpent-2-yl)benzene with hypochlorite produces 1,4-bis(3′-carboxy-2′-methylbut-2-yl)benzene and 3-(4′-carboxyphenyl)-3,3-dimethylpropanoic acid. Cyclization of this mixture forms 3,3,7,7-tetramethyl-1,2,3,5,6,7-hexahydro-s-indacen-1,5-dione, 3,3,7,7-tetramethyl-1,2,3,5,6,7-hexahydro-as-indacen-1,5-dione (5) and 6-carboxy-3,3-dimethyl-1-indanone (6). Ketoacid (6) is converted to the arylpyran pseudoacid 7-carboxy-3-hydroxy-4,4-dimethylisobenzopyran-1-one (7). In the crystal structure of (7), carboxylic acid and the pseudoacid groups each form complementary dimer hydrogen bonds linking the molecules in chains. Contact O···O distances reflect their differing energetics, with pseudoacyl O···O at 2.78(1)Å and carboxylic O···O at 2.62(1)Å.
Graphical Abstract
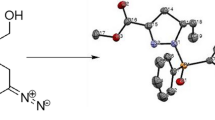
A normal, pseudodiacid shows complementary hydrogen bonds between the two similar functions forming chains.






Similar content being viewed by others
References
Leiserowitz L (1976) Acta Crystallogr B32:775
Jeffrey GA, Saenger W (1991) Hydrogen bonding in biological structures. Springer, New York
Liskin DV, Valente EJ (2008) J Mol Struct 878:149
Sheldrick GM, Kruger C, Goddard R (eds) (1985) Crystallographic computing, vol 3. Clarendon Press, Oxford, pp 175–189
Sheldrick GM (1998) Programs for crystal structure analysis (Release 97-2), Institüt für Anorganische Chemie der Universität, Tammanstrasse 4, D-3400 Göttingen, Germany
Allen FH, Samuel Motherwell WD, Raithby PR, Shields GP, Taylor R (1999) New J. Chem 23:25–34
Etter MC, MacDonald JC, Bernstein J (1990) Acta Crystallogr B46:256
Cooper WJ, Smith TN, Barker AK, Webb JA, Valente EJ (2003) J Chem Crystallogr 33:373
Valente EJ, Fuller JF, Ball JD (1998) Acta Crystallogr B54:162
Bailey M, Brown CJ (1967) Acta Crystallogr 22:387
Alcala R, Martinez-Carera S (1972) Acta Crystallogr B28:1671
Derissen JL (1974) Acta Crystallogr B30:2764
Acknowledgments
The authors thank the National Science Foundation (MRI-0618148) for resources supporting diffraction equipment and the W. M. Keck Foundation for resources supporting spectroscopy.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Milling, M.L., Cooley, J., Liskin, D.V. et al. Synthesis and structure of an arylpyran normal, pseudodiacid showing recognition. Struct Chem 20, 969–973 (2009). https://doi.org/10.1007/s11224-009-9499-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-009-9499-8