Abstract
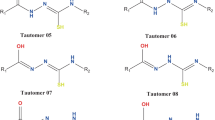
The aqueous phase AM1, PM3, and PM5 calculation data had indicated that when a potentially tautomeric amino group is placed at 3C position of the indazole ring the ring-chain tautomerism becomes feasible. However, when the amino group is placed at 4–7C of the indazole ring only the annular tautomerism was found to be feasible and no effect of amino group to provoke a ring chain tautomerism was observed. On the other hand amino form of 3 amino substituted indazole was found to be predominant over imino forms whereas for the 4–7 amino substituted indazoles imino forms were found to be predominant over amino forms. The attempt to apply soft–hard base and soft nucleophile–electrophile criteria to protonation and tautomerism phenomena was successful.






Similar content being viewed by others
References
Formosinho SJ, Arnaut L (1993) J Phtotochem Photobiol A: Chem 75:21
Scheiner S (2000) J Phys Chem A 104:5898
Wang H, Zhang H, Abou-Zeid OK, Yu C, Romesberg FE, Glasbeek M (2003) Chem Phys Lett 367:599
Ibanez GA, Labadié G, Escandar GM, Olivieri AC (2003) J Mol Struct 645:61
Knowles JR (1975) CRC Crit Rev Biochem 3:165
Hodoscek M, Kocjan D, Hadzı D (1988) J Mol Struct (THEOCHEM) 165:115–124
Lim C, Bashford D, Karplus M (1991) J Phys Chem 95:5610– 5620
Öğretir C, Kaypak NF (2002) J Mol Struct (THEOCHEM) 583:137–144
Öğretir C, Tay (Kaypak), NF (2002) J Mol Struct (THEOCHEM) 588:145–153
Catalan J, dePaz JLG, Elguero J (1995) J Chem Soc Perkin Trans 2:57–60
Stewart JJP, MOPAC 7.0 QCPE. University Indiana, Bloomington, USA
Stewart JJP, MOPAC 2002 implemented in Cache Work system Pro Version 6.1. Fujitsu Ltd
Klamt A, Schüürmann G, (1993) J Chem Soc Perkin Trans 2:799
CS ChemOffice Pro for Microsoft Windows. Cambridge Scientific Computing Inc. Cambridge, MA 02139, USA
Elguero J, Marzin C, Katritzky AR (1976) In: Katritzky AR, Boulton AJ (eds) . Advances in heterocyclic chemistry, Supplement 1. Academic, London, pp 26–27
Speranza M (1985) Adv Heterocycle Chem 40:25–104
Hudson RF, Klopman G (1967) Tetrahedron Lett 1103
Klopman G (ed) (1974) Chemical reactivity and reaction paths. Wiley, New York
Fleming I (1976) Frontier orbitals and organic reactions. Wiley, London, pp 35–40
Hemmateenejad B, Safarpour MA, Taghavi F (2003) J Mol Struct (THEOCHEM) 635:183–190
Acknowledgment
We would like to thank Prof. N. Hadjiliadis of Iaonnina University for providing us the CACHE program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Öğretir, C., Tay, N.F. Quantum chemical studies on the tautomerism of some potentially tautomeric aminoindazole derivatives. Struct Chem 17, 263–274 (2006). https://doi.org/10.1007/s11224-006-9019-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-006-9019-z