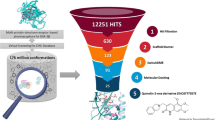
Abstract
Five scaffolds (phenoxybenzene, benzofuran, azolobenzimidazole, oxoindole, and uracil), which are promising as sources of compounds with high RAGE inhibitory activity, were found in silico among ten structurally different classes of the synthesized substances using a multitarget neural network model of the dependence of the RAGE inhibitory activity on the affinity of the compounds to the target proteins of the RAGE—NF-κB signal pathway. The derivatives of these scaffolds are characterized by the predominantly post-receptor effect and manifest different target activity spectra to signal kinases of the RAGE—NF-κB chain. The revealed multitarget compounds were recommended for experimental investigation in order to determine their RAGE inhibitory activity. They can form a basis for the development of new promising drugs for the treatment of complications after diabetes mellitus and Alzheimer’s disease.
Similar content being viewed by others
References
N. A. Ansari, Z. Rasheed, Biochemistry (Moscow), Suppl. Ser. B: Biomed. Chem., 2009, 3, No. 4, 335.
KEGG: AGE-RAGE Signaling Pathway in Diabetic Complications — Homo Sapiens, 2019; https://www.genome.jp/kegg-bin/show_pathway?map=hsa04933&show_description=show.
J. C. Tobon-Velasco, E. Cuevas, M. A. Torres-Ramos, CNS Neurol. Disord. Drug Targets, 2014, 13, 1615.
S. F. Yan, R. Ramasamy, A. M. Schmidt, Nat. Clin. Pract. Endocrinol. Metab., 2008, 4, № 5, 285.
World Health Organization: Diabetes, 2019; https://www.who.int/news-room/fact-sheets/detail/diabetes.
World Health Organization: Dementia, 2019; https://www.who.int/news-room/fact-sheets/detail/dementia.
P. M. Vassiliev, A. A. Spasov, L. R. Yanalieva, A. N. Kochetkov, V. V. Vorfolomeyeva, V. G. Klochkov, D. T. Appazova, Biochemistry (Moscow), Suppl. Ser. B: Biomed. Chem., 2019, 13, No. 3, 256.
A. A. Spasov, Yu. V. Popov, V. S. Lobasenko, T. K. Korchagina, P. M. Vassiliev, V. A. Kuznetsova, A. A. Brigadirova, A. I. Rashchenko, D. A. Babkov, A. N. Kochetkov, A. I. Kovaleva, O. S. Efremova, Russ. J. Bioorg. Chem., 2017, 43, No. 2, 163.
A. A. Spasov, D. A. Babkov, T. Y. Prokhorova, E. A. Sturova, D. R. Muleeva, M. R. Demidov, D. V. Osipov, V. A. Osyanin, Y. N. Klimochkin, Chem. Biol. Drug Des., 2017, 90, 1184.
A. A. Spasov, D. A. Babkov, D. V. Osipov, V. G. Klochkov, D. R. Prilepskay, M. R. Demidov, V. A. Osyanin, Yu. N. Klimochkin, Bioorg. Med. Chem. Lett., 2019, 29, No. 1, 119.
A. A. Spasov, O. N. Zhukovskaya, A. A. Brigadirova, H. S. A. Abbas, V. A. Anisimova, V. A. Sysoeva, A. I. Rashchenko, R. A. Litvinov, O. Yu. Mayka, D. A. Babkov, A. S. Morkovnik, Russ. Chem. Bull., 2017, 66, 1905.
D. A. Babkov, O. N. Zhukowskaya, A. V. Borisov, V. A. Babkova, E. V. Sokolova, A. A. Brigadirova, R. A. Litvinov, A. A. Kolodina, A. S. Morkovnik, V. S. Sochnev, G. S. Borodkin, A. A. Spasov, Bioorg. Med. Chem. Lett., 2019, 29, 2443.
N. A. Lozinskaya, D. A. Babkov, E. V. Zaryanova, E. N. Bezsonova, A. M. Efremov, M. D. Tsymlyakov, L. V. Anikina, O. Y. Zakharyascheva, A. V. Borisov, V. N. Perfilova, I. N. Tyurenkov, M. V. Proskurnina, A. A. Spasov, Bioorg. Med. Chem., 2019, 27, 1804.
A. A. Spasov, F. A. Khaliullin, D. A. Babkov, G. A. Timirkhanova, V. A. Kuznetsova, L. V. Naumenko, D. R. Muleeva, O. Yu. Maika, T. Yu. Prokhorova, E. A. Sturova, Pharm. Chem. J., 2017, 51, No. 7, 533.
V. L. Rusinov, I. M. Sapozhnikova, A. M. Bliznik, O. N. Chupakhin, V. N. Charushin, A. A. Spasov, P. M. Vassiliev, V. A. Kuznetsova, A. I. Rashchenko, D. A. Babkov, Arch. Pharm. (Weinheim), 2017, 350, e1600361.
A. A. Spasov, D. A. Babkov, V. A. Sysoeva, R. A. Litvinov, D. D. Shamshina, E. N. Ulomsky, K. V. Savateev, V. V. Fedotov, P. A. Slepukhin, O. N. Chupakhin, V. N. Charushin, V. L. Rusinov, Arch. Pharm. (Weinheim), 2017, 350, e1700226.
A. A. Spasov, A. K. Brel, R. A. Litvinov, S. V. Lisina, A. F. Kucheryavenko, Yu. N. Budaeva, O. A. Salaznikova, A. I. Rashchenko, D. D. Shamshina, V. V. Batrakov, A. V. Ivanov, Russ. J. Bioorg. Chem., 2018, 44, No. 6, 769.
M. S. Dzyurkevich, D. A. Babkov, N. V. Shtyrlin, O. Y. Mayka, A. G. Iksanova, P. M. Vassiliev, K. V. Balakin, A. A. Spasov, V. V. Tarasov, G. Barreto, Y. G. Shtyrlin, G. Aliev, Sci. Rep., 2017, 7, 16072.
ChemAxon: Marvin, 2019; https://chemaxon.com/products/marvin.
MOPAC, 2019, http://openmopac.net.
UniProt: UniProtKB, 2019; https://www.uniprot.org/uniprot/.
Statistica, 2007; http://www.statsoft.com/Products/STATISTICA-Features.
R. I. Ishmetova, D. A. Babkov, A. F. Kucheryavenko, V. A. Babkova, V. S. Sirotenko, N. K. Ignatenko, S. G. Tolschina, P. M. Vassiliev, G. L. Rusinov, A. A. Spasov, Russ. Chem. Bull., 2020, 69, 768.
A. A. Spasov, O. N. Zhukowskaya, D. A. Babkov, A. A. Brigadirova, V. A. Babkova, A. S. Morkovnik, R. A. Litvinov, E. V. Sokolova, Russ. Chem. Bull., 2020, 69, 774.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was financially supported by the Russian Foundation for Basic Research (Project No. 18-015-00499).
This work does not involve human participants and animal subjects.
The authors declare that there is no conflict of interest.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 3, pp. 562–566, March, 2021.
Rights and permissions
About this article
Cite this article
Vassiliev, P.M., Spasov, A.A., Babkov, D.A. et al. Neural network modeling search for multitarget RAGE inhibitors with different target activity spectra. Russ Chem Bull 70, 562–566 (2021). https://doi.org/10.1007/s11172-021-3125-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-021-3125-3