Abstract
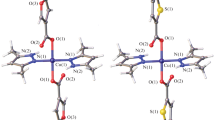
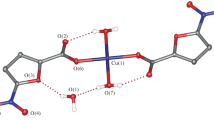
The reaction of copper(ii) acetate with 2-furancarboxylate (Hfur, pyromucate) anions and the N-donor ligands 4-phenylpyridine (phpy) and 3-aminopyridine (NH2py) in acetonitrile afforded the mononuclear complexes of the composition [Cu(fur)2(phpy)2(H2O)] · phpy (1) and [Cu(fur)2(NH2py)2] (2), respectively. The structures of the complexes were established by X-ray diffraction. The simultaneous thermal analysis of the thermal behavior of complex 1 showed that this complex is thermally stable up to 125 °C. The in vitro biological activity of complexes 1 and 2 was evaluated against the non-pathogenic mycobacterial Mycolicibacterium smegmatis strain.
Similar content being viewed by others
References
M. M. Rashad, O. A. Fouad, Mater. Chem. Phys., 2005, 94, 365.
M. K. Karunananda, F. X. Vázquez, E. E. Alp, W. Bi, S. Chattopadhyay, T. Shibatad, N. P. Mankad, Dalton Trans., 2014, 43, 13661.
M. Veith, M. Haas, V. Huch, Chem. Mater., 2005, 17, 95.
R. V. Godbole, P. Rao, P. S. Alegaonkar, S. Bhagwat, Mater. Chem. Phys., 2015, 161, 135.
L. W. Yeary, J. W. Moon, C. J. Rawn, L. J. Love, A. J. Rondinone, J. R. Thompson, B. C. Chakoumakos, T. J. Phelps, J. Magn. Magn. Mater., 2011, 323, 3043.
A. Y. Louie, T. Meade, J. Proc. Natl. Acad. Sci. USA, 1998, 95, 6663.
S. Rojas, E. Quartapelle-Procopio, F. J. Carmona, F. J. Carmona, M. A. Romero, J. A. R. Navarro, E. Barea, J. Mater. Chem. B, 2014, 2, 2473.
K. H. Thompson, C. J. Orvig, Inorg. Biochem., 2006, 100, 1925.
N. Bello-Vieda, H. Pastrana, M. Garavito, A. Ávila, A. Celis, A. Muñoz-Castro, S. Restrepo, J. J. Hurtado, Molecules, 2018, 23, 361.
L. Goodwin, Trop. Med. Hyg., 1995, 89, 339.
A. Singh, A. K. Gupta, S. Singh, Nano Bio Medicine, 2020, 1.
A. S. Brill, J. Biochem. Mol. Biol. Biophys., 1977, 26, 1.
P. M. Punt, G. H. Clever, Chemistry, 2019, 25, 13987.
C. L. Drennan, J. W. Peters, Curr. Opin. Struct. Biol., 2003, 13, 220.
T. S. Coelho, P. C. B. Halicki, L. Silva de Menezes, J. R. Vicenti, B. L. Gonçalves, P. E. Almeida da Silva, D. F. Ramos, Lett. Appl. Microbiol., 2020, 71, 146.
N. Chim, P. M. Johnson, C. W. Goulding, J. Inorg. Biochem., 2014, 133, 118.
O. Krasnovskaya, A. Naumov, D. Guk, P. Gorelkin, A. Erofeev, E. Beloglazkina, A. Majouga, Int. J. Mol. Sci., 2020, 21, 3695.
J. P. Rada, B. S. M. Bastos, L. Anselmino, C. H. J. Franco, M. Lanznaster, R. Diniz, C. O. Fernández, M. Menacho-Márquez, A. M. Percebom, N. A. Rey, Inorg. Chem., 2019, 58, 8800.
K. Y. Djoko, C. L. Ong, M. J. Walker, A. G. McEwan, J. Biol. Chem., 2015, 290, 18954.
Yu. A. Ovchinnikov, Bioorganicheskaya khimiya [Bioorganic Chemistry], Prosveshchenie, Moscow, 1987, 516 pp. (in Russian).
A. H. Ngwane, R. D. Petersen, B. Baker, I. Wiid, H. N. Wong, R. K. Haynes, IUBMB Life, 2019, 71, 532.
I. A. Lutsenko, D. E. Baravikov, M. A. Kiskin, Yu. V. Nelyubina, P. V. Primakov, O. B. Bekker, A. V. Khoroshilov, A. A. Sidorov, I. L. Eremenko, Russ. J. Coord. Chem., 2020, 46, 411.
I. A. Lutsenko, D. S. Yambulatov, M. A. Kiskin, Yu. V. Nelyubina, P. V. Primakov, O. B. Bekker, A. A. Sidorov, I. L. Eremenko, Russ. J. Coord. Chem., 2020, 46, 787.
I. A. Lutsenko, D. S. Yambulatov, M. A. Kiskin, Yu. V. Nelyubina, P. V. Primakov, O. B. Bekker, O. A. Levitskiy, T. V. Magdesieva, V. K. Imshennik, Yu. V. Maksimov, A. A. Sidorov, V. N. Danilenko, I. L. Eremenko, Chem. Select., 2020, 5, 11837.
I. A. Lutsenko, M. A. Kiskin, Yu. V. Nelyubina, P. V. Primakov, M. A. Shmelev, N. N. Efimov, K. S. Babeshkin, A. V. Khoroshilov, A. A. Sidorov, I. L. Eremenko, Polyhedron, 2020, 190, 114764.
S. Ramon-García, C. Ng, H. Anderson, J. D. Chao, X. Zheng, T. Pfeifer, Y. Av-Gay, M. Roberge, C. J. Thompson, Antimikrob. Agen. Chemother., 2011, 8, 3861.
O. B. Bekker, D. N. Sokolov, O. A. Luzina, N. I. Komarova, Yu. V. Gatilov, S. N. Andreevskaya, T. G. Smirnova, D. A. Maslov, L. N. Chernousova, N. F. Salakhutdinov, V. N. Danilenko, Med. Chem. Res., 2015, 24, 2926.
G. M. Sheldrick, Acta Cryst., 2015, A71, 3.
O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, H. Puschmann, J. Appl. Cryst., 2009, 42, 339.
Author information
Authors and Affiliations
Corresponding author
Additional information
The work was financially supported by the Russian Science Foundation (Project No. 20-13-00061).
Elemental analysis, IR spectroscopy measurements, and STA were performed using equipment of the Shared Facility Center for Physical Research Methods of Substances and Materials of the N. S. Kurnakov Institute of General and Inorganic Chemistry of the Russian Academy of Sciences. Single-crystal X-ray diffraction analysis of complexes 1 and 2 was carried out with the financial support from the Ministry of Science and Higher Education of the Russian Federation using equipment of the Center for Molecular Composition Studies of the A. N. Nesmeyanov Institute of Organoelement Compounds of the Russian Academy of Sciences.
No human or animals were involved in this research.
The authors declare no conflict of interest, financial or otherwise.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 3, pp. 463–468, March, 2021.
Rights and permissions
About this article
Cite this article
Lutsenko, I.A., Kiskin, M.A., Koshenskova, K.A. et al. Synthesis, structure, and in vitro evaluation of biological activity of CuII furancarboxylates against the non-pathogenic M. smegmatis strain. Russ Chem Bull 70, 463–468 (2021). https://doi.org/10.1007/s11172-021-3109-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-021-3109-3