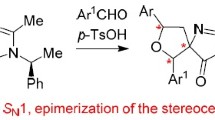
Abstract
Reaction of donor-acceptor cyclopropanes with 1,3-diphenylisobenzofuran in the presence of lanthanide triflates, as well as CuOTf, Sn(OTf)2, SnCl2, ZnCl2, GaCl3, and MgI2, proceeds as a formal [3+4]-cycloaddition leading to a newly formed seven-membered ring. This reaction was found to be typical of cyclopropane-1,1-diesters and dinitriles, as well as 1-nitrocyclo-propanecarboxylates containing aromatic, heteroaromatic, and vinylic substituents at the C(2) atom of the small ring. When Me3SiOTf, TiCl4, SnCl4, or BF3·OEt2 were used as initiators, unusual cyclic hemiacetals were formed via the conjugate 1,4-addition of a cyclopropane and a nucleophile to the diene moiety.
Similar content being viewed by others
References
H.-U. Reissig, R. Zimmer, Chem. Rev., 2003, 103, 1151.
M. Yu, B. L. Pagenkopf, Tetrahedron, 2005, 61, 321.
D. Agrawal, V. K. Yadav, Chem. Commun., 2008, 6471.
C. A. Carson, M. A. Kerr, Chem. Soc. Rev., 2009, 38, 3051.
F. De Simone, J. Waser, Synthesis, 2009, 3353.
M. Ya. Mel’nikov, E. M. Budynina, O. A. Ivanova, I. V. Trushkov, Mendeleev Commun., 2011, 21, 293.
P. Tang, Y. Qin, Synthesis, 2012, 2969.
Z. Wang, Synlett, 2012, 2311.
S. Haubenreisser, P. Hensenne, S. Schroeder, M. Niggemann, Org. Lett., 2013, 15, 2262.
G. Yang, Y. Sun, Y. Shen, Z. Chai, S. Zhou, J. Chu, J. Chai, J. Org. Chem., 2013, doi: 10.1021/jo400554a.
Y. Miyake, S. Endo, T. Moriyama, K. Sakata, Y. Nishibayashi, Angew. Chem., Int. Ed., 2013, 52, 1758.
W. Zhu, J. Fang, Y. Liu, J. Ren, Z. Wang, Angew. Chem., Int. Ed., 2013, 52, 2032.
Yu. A. Volkova, E. M. Budynina, A. E. Kaplun, O. A. Ivanova, A. O. Chagarovskiy, D. A. Skvortsov, V. B. Rybakov, I. V. Trushkov, M. Ya. Melnikov, Chem. Eur. J., 2013, 19, 6586.
H. Xiong, H. Xu, S. Liao, Z. Xie, Y. Tang, J. Am. Chem. Soc., 2013, 135, 7851.
H. Xu, J.-P. Qu, S. Liao, H. Xiong, Y. Tang, Angew. Chem., Int. Ed., 2013, 52, 4004.
X.-F. Xia, X.-R. Song, X.-Y. Liu, Y.-M. Liang, Chem. Asian J., 2012, 7, 1538.
E. O. Gorbacheva, A. A. Tabolin, R. A. Novikov, Yu. A. Khomutova, Yu. V. Nelyubina, Yu. V. Tomilov, S. L. Ioffe, Org. Lett., 2013, 15, 350.
Y.-Y. Zhou, J. Li, L. Ling, S.-H. Liao, X.-L. Sun, Y.-X. Li, L.-J. Wang, Y. Tang, Angew. Chem., Int. Ed., 2013, 52, 1452.
W. J. Humenny, P. Kyriacou, K. Sapeta, A. Karadeolian, M. A. Kerr, Angew. Chem., Int. Ed., 2012, 51, 11088.
O. A. Ivanova, E. M. Budynina, Yu. K. Grishin, I. V. Trushkov, P. V. Verteletskii, Angew. Chem., Int. Ed., 2008, 47, 1107.
O. A. Ivanova, E. M. Budynina, Yu. K. Grishin, I. V. Trushkov, P. V. Verteletskii, Eur. J. Org. Chem., 2008, 5329.
O. A. Ivanova, E. M. Budynina, A. O. Chagarovskiy, A. E. Kaplun, I. V. Trushkov, M. Ya. Melnikov, Adv. Synth. Catal., 2011, 353, 1125.
A. O. Chagarovskiy, Ph.D. Thesis (Chem.), Department of Chemistry, M. V. Lomonosov Moscow State University, Moscow, 2011, 168 pp. (in Russian).
A. O. Chagarovskiy, E. M. Budynina, O. A. Ivanova, Yu. K. Grishin, I. V. Trushkov, P. V. Verteletskii, Tetrahedron, 2009, 65, 5385.
J. Fang, J. Ren, Z. Wang, Tetrahedron Lett., 2008, 49, 6659.
J.-P. Qu, C. Deng, J. Zhou, X.-L. Sun, Y. Tang, J. Org. Chem., 2009, 74, 7684.
P. E. O’Bannon, W. P. Dailey, Tetrahedron, 1990, 46, 7341.
O. Lifchits, A. B. Charette, Org. Lett., 2008, 10, 2809.
O. Lifchits, D. Alberico, I. Zakharian, A. B. Charette, J. Org. Chem., 2008, 73, 6838.
V. N. G. Lindsay, C. Nicolas, A. B. Charette, J. Am. Chem. Soc., 2011, 133, 8972.
S. S. So, T. J. Auvil, V. J. Garza, A. E. Mattson, Org. Lett., 2012, 14, 444.
R. Shintani, M. Murakami, T. Tsuji, H. Tanno, T. Hayashi, Org. Lett., 2009, 11, 5642.
M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, Jr., R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, P. Salvador, J. J. Dannenberg, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, A. G. Baboul, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, M. Challacombe, P. M. W. Gill, B. Johnson, W. Chen, M. W. Wong, J. L. Andres, C. Gonzalez, M. Head-Gordon, E. S. Replogle, J. A. Pople, Gaussian 98, Revision A.11, Gaussian, Inc., Pittsburgh (PA), 2001.
D. O. Jang, D. D. Kim, D. K. Pyun, P. Beak, Org. Lett., 2003, 5, 4155.
P. D. Pohlhaus, S. D. Sanders, A. T. Parsons, W. Li, J. S. Johnson, J. Am. Chem. Soc., 2008, 130, 8642.
M. J. Campbell, J. S. Johnson, A. T. Parsons, P. D. Pohlhaus, S. D. Sanders, J. Org. Chem., 2010, 75, 6317.
A. Karadeolian, M. A. Kerr, J. Org. Chem., 2007, 72, 10251.
C. Perreault, S. R. Goudreau, L. E. Zimmer, A. B. Charette, Org. Lett., 2008, 10, 689.
A. Sliwinska, W. Czardybon, J. Warkentin, Org. Lett., 2007, 9, 695.
M. J. Alves, N. G. Azoia, J. F. Bickley, A. G. Fortes, T. L. Gilchrist, R. Mendonca, J. Chem. Soc., Perkin Trans. 1, 2001, 2969.
M. J. Alves, N. G. Azoia, A. G. Fortes, Heterocycles, 2005, 65, 1329.
E. J. Corey, M. Chaykovsky, J. Am. Chem. Soc., 1965, 86, 1353.
W. Fraser, C. J. Suckling, H. C. S. Wood, J. Chem. Soc., Perkin Trans. 1, 1990, 3137.
G. M. Sheldrick, Acta Crystallogr., 2008, A64, 112.
A. L. Spek, J. Appl. Crystallogr., 2003, 36, 7.
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to Academician of the Russian Academy of Sciences M. P. Egorov on the occasion of his 60th birthday.
Published in Russian in Izvestiya Akademii Nauk. Seriya Khimicheskaya, No. 11, pp. 2407–2423, Novemer, 2013.
Rights and permissions
About this article
Cite this article
Chagarovskiy, A.O., Ivanova, O.A., Budynina, E.M. et al. Reaction of donor-acceptor cyclopropanes with 1,3-diphenylisobenzofuran. Lewis acid effect on the reaction pathway. Russ Chem Bull 62, 2407–2423 (2013). https://doi.org/10.1007/s11172-013-0349-x
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11172-013-0349-x