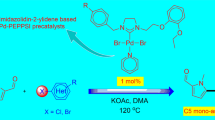
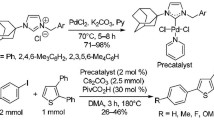
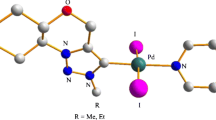
Abstract
N-Heterocyclic carbene (NHC)-linked PEPPSI-type palladium complexes have recently been used in the direct C-H bond arylation of heteroarenes. However, in most of the published works, NHC ligands containing benzimidazole and imidazole ring have been used, but using NHC ligands containing saturated imidazoline ring is quite rare. Therefore, in this study, four new 1,3-disubstituted imidazolinium salts as NHC ligand precursors, and their four new PEPPSI-type palladium complexes were synthesized. The structures of all new compounds were fully characterized by different spectroscopic and analytical techniques. The more detailed structural characterization of one of the palladium complexes was determined by single-crystal X-ray diffraction study. The catalytic activities of all palladium complexes were evaluated in the direct C-H bond arylation of the 2-acetylfuran and 2-acetylthiophene with (hetero) aryl bromides and readily available and inexpensive aryl chlorides in presence of 1 mol% catalyst loading at 120 °C. Under the given conditions, (hetero)aryl halides were successfully applied as the arylating reagents to achieve the C5-arylated furans and thiophenes in acceptable to high yields.
Graphic abstract




Similar content being viewed by others
References
W.A. Herrmann, C. Köcher, Angew. Chem. Int. Ed. Engl. 36, 2162 (1997)
W.A. Herrmann, Angew. Chem. Int. Ed. 41, 1290 (2002)
F.E. Hahn, M.C. Jahnke, Angew. Chem. Int. Ed. 47, 3122 (2008)
M.N. Hopkinson, C. Richter, M. Schedler, F. Glorius, Nature 510, 485 (2014)
D.J. Nelson, Eur. J. Inorg. Chem. 12, 2012 (2015)
S.-Z. Wu, Q. Yu, Y.-H. Li, G.-H. Cui, Res. Chem. Intermed. 47, 835 (2021)
S.P. Nolan (ed.), N-Heterocyclic Carbenes Effective Tools for Organometallic Synthesis (Wiley-VCH, Weinheim, 2014), p. 544
W.A. Herrmann, M. Elison, J. Fischer, C. Köcher, G.R.J. Artus, Angew. Chem. Int. Ed. 34, 2371 (1995)
E. Peris, Chem. Rev. 118, 9988 (2017)
S. Díez-González, N. Marion, S.P. Nolan, Chem. Rev. 109, 3612 (2009)
B. Liu, J. Li, F. Song, J. You, Chem. Eur. J. 18, 10830 (2012)
M.E. Benhalouche, H. Li, A. Miloudi, A. Benzai, M. Cordier, J.-F. Soulé, H. Doucet, Eur. J. Org. Chem. 30, 4792 (2020)
J. Yang, Appl. Organometal. Chem. 34, e5450 (2020)
B. Eftekhari-Sis (ed.), Aromatic C(Sp2)-H Dehydrogenative Coupling Reactions: Heterocycles Synthesis (CRC Press, Boca Raton, 2019)
N. Amirmahani, N.O. Mahmoodi, M. Malakootian, A. Pardakhty, N. Seyedi, Res. Chem. Intermed. 46, 4595 (2020)
F. Jafari, A. Ghorbani-Choghamarani, N. Hasanzadeh, Res. Chem. Intermed. 47, 1033 (2021)
M. Miura, T. Satoh, Palladium-catalyzed direct arylation reactions, in Modern arylation methods. ed. by L. Ackermann (Wiley-VCH, Weinheim, 2009), pp. 335–361
J.-Q. Yu, Z. Shi (eds.), C-H Activation Topics in Current Chemistry (Springer, Berlin, Heidelberg, 2010)
X. Shi, E.D.S. Carrizo, M. Cordier, J. Roger, N. Pirio, J.-C. Hierso, P. Fleurat-Lessard, J.-F. Soulé, H. Doucet, Chem. Eur. J. 27, 1 (2021)
V. Dimakos, M.S. Taylor, Org. Biomol. Chem. 19, 514 (2021)
Y. Kitamura, Y. Murata, M. Iwai, M. Matsumura, S. Yasuike, Molecules 26, 97 (2021)
F. Bellina, R. Rossi, Tetrahedron 65, 10259 (2009)
M. Kaloğlu, N. Kaloğlu, İ Yıldırım, N. Özdemir, İ Özdemir, J. Mol. Struc. 1206, 127668 (2020)
M. Kaloglu, S. Demir Dusunceli, I. Ozdemi, J. Organometal. Chem. 915, 121236 (2020)
S. Sreekumar, S. Xavier, A. Govindan, R.E. Thampikannu, K. Vellayan, B. González, Res. Chem. Intermed. 46, 4529 (2020)
C.B. Bheeter, L. Chen, J.-F. Soulé, H. Doucet, Catal. Sci. Technol. 6, 2005 (2016)
I.V. Seregin, V. Gevorgyan, Chem. Soc. Rev. 36, 1173 (2007)
D. Alberico, M.E. Scott, M. Lautens, Chem. Rev. 107, 174 (2007)
L.-C. Campeau, D.R. Stuart, K. Fagnou, Aldrichimica Acta 40, 35 (2007)
G.P. McGlacken, L.M. Bateman, Chem. Soc. Rev. 38, 2447 (2009)
L. Ackermann, R. Vicente, A.R. Kapdi, Angew. Chem. Int. Ed. 48, 9792 (2009)
L. Ackermann, Chem. Rev. 111, 1315 (2011)
N. Nakamura, Y. Tajima, K. Sakai, Heterocycles 17, 235 (1982)
A. Ohta, Y. Akita, T. Ohkuwa, M. Chiba, R. Fukunaga, A. Miyafuji, T. Nakata, N. Tani, Y. Aoyagi, Heterocycles 31, 1951 (1990)
I.A. Stepek, K. Itami, ACS Materials Lett. 2, 951 (2020)
C.J. O’Brien, E.A.B. Kantchev, C. Valente, N. Hadei, G.A. Chass, A. Lough, A.C. Hopkinson, M.G. Organ, Chem. Eur. J. 12, 4743 (2006)
F. Glorius (ed.), N-Heterocyclic Carbenes in Transition Metal Catalysis (Springer-Verlag, Berlin, 2007)
C. Valente, S. Çalimsiz, K.H. Hoi, D. Mallik, M. Sayah, M.G. Organ, Angew. Chem. Int. Ed. 51, 3314 (2012)
Q. Zhao, G. Meng, S.P. Nolan, M. Szostak, Chem. Rev. 120, 1981 (2020)
X.-X. He, Y. Li, B.B. Ma, Z. Ke, F.S. Liu, Organometallics 35, 2655 (2016)
L.Q. Hu, R.L. Deng, Y.F. Li, C.J. Zeng, D.S. Shen, F.S. Liu, Organometallics 37, 214 (2018)
A.R. Martin, A. Chartoire, A.M.Z. Slawin, S.P. Nolan, Beilstein J. Org. Chem. 8, 1637 (2012)
E.D. Coy, L.E. Cuca, M. Sefkow, Synth. Commun. 41, 41 (2010)
K.-T. Chan, Y.-H. Tsai, W.-S. Lin, J.-R. Wu, S.-J. Chen, F.-X. Liao, C.-H. Hu, H.M. Lee, Organometallics 29, 463 (2010)
S. Otsuka, H. Yorimitsu, A. Osuka, Chem. Eur. J. 21, 14703 (2015)
W. Chen, J. Yang, J. Organometal. Chem. 872, 24 (2018)
M. Kaloğlu, İ Özdemir, Inorg. Chim. Acta 504, 119454 (2020)
M. Kaloğlu, N. Kaloğlu, İ Özdemir, Catal. Lett. (2021).
L. Yang, D.R. Powell, R.P. Houser, Dalton Trans. 9, 955 (2007)
A. Okuniewski, D. Rosiak, J. Chojnacki, B. Becker, Polyhedron 90, 47 (2015)
B. Cordero, V. Gomez, A.E. Platero-Prats, M. Reves, J. Echeverria, E. Cremades, F. Barragan, S. Alvarez, Dalton Trans. 21, 2832 (2008)
N. Kaloğlu, M. Kaloğlu, M.N. Tahir, C. Arıcı, C. Bruneau, H. Doucet, P.H. Dixneuf, B. Çetinkaya, İ Özdemir, J. Organomet. Chem. 867, 404 (2018)
Y. Han, H.V. Huynh, G.K. Tan, Organometallics 26, 6447 (2007)
H.V. Huynh, W. Sim, C.F. Chin, Dalton Trans. 40, 11690 (2011)
Y.-C. Lin, H.-H. Hsueh, S. Kanne, L.-K. Chang, F.-C. Liu, I.J.B. Lin, G.-H. Lee, S.-M. Peng, Organometallics 32, 3859 (2013)
L. Barbu, M.M. Popa, S. Shova, M. Ferbinteanu, C. Draghici, F. Dumitrascu, Inorg. Chim. Acta 463, 97 (2017)
M. Kaloğlu, İ Özdemir, V. Dorcet, C. Bruneau, H. Doucet, Eur. J. Inorg. Chem. 10, 1382 (2017)
M.S. McClure, B. Glover, E. McSorley, E. Millar, M.H. Osterhout, F. Roschangar, Org. Lett. 3, 1677 (2001)
B. Glover, K.A. Harvey, B. Liu, M.J. Sharp, M.F. Tymoschenko, Org. Lett. 5, 301 (2003)
M. Parisien, D. Valette, K. Fagnou, J. Org. Chem. 70, 7578 (2005)
E. David, C. Rangheard, S. Pellet-Rostaing, M. Lemaire, Synlett 13, 2016 (2006)
E. David, S. Pellet-Rostaing, E. Lemaire, Tetrahedron 63, 8999 (2007)
A. Battace, M. Lemhadri, T. Zair, H. Doucet, M. Santelli, Organometallics 26, 472 (2007)
H.A. Chiong, O. Daugulis, Org. Lett. 9, 1449 (2007)
A. Battace, M. Lemhadri, T. Zair, H. Doucet, M. Santelli, Adv. Synth. Catal. 349, 2507 (2007)
P. Amaladass, J.A. Clement, A.K. Mohanakrishnan, Tetrahedron 63, 10363 (2007)
F. Derridj, A.L. Gottumukkala, S. Djebbar, H. Doucet, Eur. J. Inorg. Chem. 16, 2550 (2008)
F. Pozgan, J. Roger, H. Doucet, Chemsuschem 1, 404 (2008)
J. Roger, F. Pozgan, H. Doucet, Green Chem. 11, 425 (2009)
M. Kaloğlu, İ Özdemir, Appl. Organometal. Chem. 32, e4399 (2018)
M. Kaloğlu, N. Gürbüz, İ. Yıldırım, N. Özdemir, İ. Özdemir, Appl. Organometal. Chem. 34, e5387 (2020)
X-AREA (Version 1.18) and X-RED32 (Version 1.04), Stoe & Cie, (Darmstadt, Germany, (2002)
L. Palatinus, G. Chapuis, J. Appl. Crystallogr. 40, 786 (2007)
G.M. Sheldrick, Acta Crystallogr. C71, 3 (2015)
O.V. Dolomanov, L.J. Bourhis, R.J. Gildea, J.A.K. Howard, H. Puschmann, J. Appl. Crystallogr. 42, 339 (2009)
Acknowledgements
This study was supported by the Technological and Scientific Research Council of Turkey TÜBİTAK (Project No: 119R003) and Ondokuz Mayıs University (Project No: PYO.FEN.1906.19.001).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix CCDC 2,050,747 for the complex 4d contains the supplementary crystallographic data for the compound reported in this article. These data can be obtained free of charge on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK [E-mail: deposit@ccdc.cam.ac.uk, https://www.ccdc.cam.ac.uk/structures/].
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kaloğlu, M., Kaloğlu, N., Özdemir, N. et al. The first use of [PdBr2(imidazolidin-2-ylidene)(pyridine)] catalysts in the direct C-H bond arylation of C2-substituted furan and thiophene. Res Chem Intermed 47, 2821–2843 (2021). https://doi.org/10.1007/s11164-021-04444-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-021-04444-4