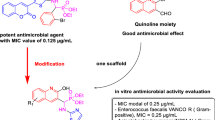
Abstract
In this manuscript we have demonstrated a new approach for the synthesis of 2-amino-4H-chromen-4-ylphosphonates and β-phosphonomalonates linked 2-chloroquinoline-3-carbaldehyde by modified one-pot three-component tandem Knoevenagel–Phospha-Michael reaction of salicylaldehyde/aryl aldehyde/2-chloroquinoline-3-carbaldehyde, malononitrile/ethylcyanoacetate, and phosphite ester using triethylamine (1–10 mol%) in ethanol under reflux conditions. The desired products were obtained in 86–97% yield in 8–35 h. The advantages of this protocol are its operational simplicity, low catalytic loading, no side product formation, and high yield of product. The newly synthesized β-phosphonomalonates, diethyl-(2-chloroquinolin-3-yl)-2,2-dicyanoethyl)-phosphonates (4a–i) have been tested on two fungal strains (C. albicans and A. fumigatus) and two bacterial strains (S. aureus and E. coli) and their minimum inhibitory concentration was also determined by microbroth dilution method.


Similar content being viewed by others
References
R.P. Herrera, E. Marques-Lopez, Multi-component Reactions: Concepts and Applications for Design and Synthesis (Wiley, London, 2015)
J. Zhu, Q. Wang, M. Wang (eds.), Multi-component Reactions in Organic Synthesis (Wiley, London, 2014)
J. Zhu, H. Bienayme (eds.), Multi-component Reactions (Wiley, London, 2005)
A. Domling, W. Wang, K. Wang, Chem. Rev. 112, 3083 (2012)
J.D. Sunderhaus, S.F. Martin, Chem. Eur. J. 15, 1300 (2009)
A. Dalhoff, Infection 22, S111 (1994)
V.J. Lee, S.J. Hecker, Med. Res. Rev. 19, 521 (1999)
K. Poole, Curr. Opin. Microbiol. 4, 500 (2001)
D.M. Livermore, Int. J. Antimicrob. Agents 16, 3 (2000)
D. Abbanat, M. Macielag, K. Bush, Expert Opin. Investig. Drugs 12, 379 (2003)
S.I. Pretorius, W.J. Breytenbach, C. de Kock, P.J. Smith, D.D. N’Da, Bioorg. Med. Chem. 21, 269 (2013)
V. Ramesh, B.A. Rao, P. Sharma, B. Swarna, D. Thummuri, K. Srinivas, V.G.M. Naidu, V.J. Rao, Eur. J. Med. Chem. 83, 569 (2014)
N.C. Desai, A.S. Maheta, K.M. Rajpara, V.V. Joshi, H.V. Vaghani, H.M. Satodiya, J. Saudi Chem. Soc. 18, 963 (2014)
S.J. Benkovic, S.J. Baker, M.R.K. Alley, Y.-H. Woo, Y.-K. Zhang, T. Akama, W. Mao, J. Baboval, P.T.R. Rajagopalan, M. Wall, L.S. Kahng, A. Tavassoli, L. Shapiro, J. Med. Chem. 48, 7468 (2005)
N. Saravanan, M. Arthanareeswari, P. Kamaraj, B. Sivakumar, Res. Chem. Intermed. 41, 5379 (2015)
Y.-L. Chen, I.-L. Chen, C.-M. Lu, C.-C. Tzeng, L.-T. Tsao, J.-P. Wang, Bioorg. Med. Chem. 11, 3921 (2003)
K. Kaur, M. Jain, R.P. Reddy, R. Jain, Eur. J. Med. Chem. 45, 3245 (2010)
C. Zhi, Z. Long, A. Manikowski, J. Comstock, W. Xu, N.C. Brown, P.M. Tarantino, K.A. Holm, E.J. Dix, G.E. Wright, M.H. Barnes, M.M. Butler, K.A. Foster, W.A. Lamarr, B. Bachand, R. Bethell, C. Cadilhac, S. Charron, S. Lamothe, I. Motorina, R. Storer, J. Med. Chem. 49, 1455 (2006)
A.R. Deshmukh, M.R. Bhosle, L.D. Khillare, S.T. Dhumal, A. Mishra, A.K. Srivastava, R.A. Mane, Res. Chem. Intermed. (2016). doi:10.1007/s11164-016-2686-5
V. Ramesh, B.A. Rao, P. Sharma, B. Swarna, D. Thummuri, K. Srinivas, V.G.M. Naidu, V.J. Rao, Eur. J. Med. Chem. 83, 569 (2014)
B. Stowasser, K.-H. Budt, L.J. Jian-Qi, A. Peyman, D. Ruppert, Tetrahedron Lett. 33, 6625 (1992)
M.C. Allen, W. Fuhrer, B. Tuck, R. Wade, J.M. Wood, J. Med. Chem. 32, 1652 (1989)
P. Kafarski, B. Lejczak, Phosphorus Sulphur Silicon Relat. Elem. 63, 193 (1991)
B.E. Maryanoff, A.B. Reitz, Chem. Rev. 89, 863 (1989)
M. Ough, A. Lewis, E.A. Bey, J. Gao, J.M. Ritchie, W. Bornmann, D.A. Boothman, L.W. Oberley, J.J. Cullen, Cancer Biol. Ther. 4, 95 (2005)
D.O. Moon, Y.H. Choi, N.D. Kim, Y.M. Park, G.Y. Kim, Int. Immunopharmacol. 7, 506 (2007)
P.S. Elisa, E.B. Ana, A.G. Ravelo, D.J. Yapu, A.G. Turba, Chem. Biodivers. 2, 264 (2005)
M.M. Khafagy, A.H.F.A. El-Wahas, F.A. Eid, A.M. El-Agrody, Farmaco 57, 715 (2002)
P. Jayashree, G. Shanthi, P.T. Perumal, Synlett 6, 917 (2009)
B. Das, P. Balasubramanyam, G.C. Reddy, N. Salvanna, Helv. Chim. Acta 94, 1347 (2011)
D.S. Gaikwad, K.A. Undale, T.S. Shaikh, D.M. Pore, Comptes Rendus Chim. 14, 865 (2011)
M. Rajasekhar, K.U.M. Rao, C.S. Sundar, N.B. Reddy, S.K. Nayak, C.S. Reddy, Chem. Pharm. Bull. 60, 854 (2012)
R. Mohammadi, M.Z. Kassaee, J. Mol. Catal. A: Chem. 380, 152 (2013)
R.M.N. Kalla, S.J. Byeon, M.S. Heo, I. Kim, Tetrahedron 69, 10544 (2013)
M.N. Elinson, R.F. Nasybullin, G.I. Nikishin, Heteroat. Chem. 24, 398 (2013)
G. Brahmachari, S. Laskar, Phosphorus Sulphur Silicon Relat. Elem. 189, 873 (2014)
M.A. Kulkarni, V.R. Pandurangi, U.V. Desai, P.P. Wadgaonkar, Comptes Rendus Chim. 15, 745 (2012)
S.R. Kolla, Y.R. Lee, Tetrahedron 68, 226 (2012)
S. Sobhani, M. Honarmand, Catal. Lett. 143, 476 (2013)
P. Kour, A. Kumar, V.K. Rai, Comptes Rendus Chim. 20, 140 (2017)
S. Sobhani, S. Rezazadeh, Synlett 2010, 1485 (2010)
S. Sobhani, Z.P. Parizi, S. Rezazadeh, J. Organomet. Chem. 696, 813 (2011)
S. Sobhani, Z.P. Parizi, Tetrahedron 67, 3540 (2011)
M. Hosseini-Sarvari, S. Etemad, Tetrahedron 64, 5519 (2008)
S. Sobhani, M. Bazrafshan, A.A. Delluei, Z.P. Parizi, Appl. Catal. A Gen. 454, 145 (2013)
H. Sharghi, S. Ebrahimpourmoghaddam, M.M. Doroodmand, Tetrahedron 69, 4708 (2013)
S. Sobhani, R. Jahanshahi, Synth. Commun. 43, 3247 (2013)
B.A. Dar, N. Pandey, S. Singh, R.K. Bamezai, M. Sharma, R.A. Vishwakarma, B. Singh, Tetrahedron Lett. 55, 623 (2014)
Y.-Q. Yu, D.-Z. Xu, Tetrahedron 71, 2853 (2015)
S. Sobhani, F. Zarifi, RSC. Adv. 5, 96532 (2015)
Y.-Q. Yu, D.-Z. Xu, RSC. Adv. 5, 28857 (2015)
R.M.N. Kalla, H. Park, H.R. Lee, H. Suh, I. Kim, ACS Comb. Sci. 17, 691 (2015)
R.U. Pokalwar, P.V. Shinde, A.B. Chidrawar, P.R. Ballari, B.B. Shingate, M.S. Shingare, Chem. Biol. Interface 2, 31 (2012)
S. Sobhani, Z. Pakdin-Parizi, RSC. Adv. 4, 13071 (2014)
A.A. Fahmy, N.A. Ismail, T.S. Hafez, Phosphorus Sulfur Silicon Relat. Elem. 66, 201 (1992)
O. Meth-Cohn, B. Narine, B. Tarnowski, Tetrahedron Lett. 20, 3111 (1979)
A. Kumar, S. Kumar, K.K. Kapoor, Aust. J. Chem. 60, 621 (2007)
V.K. Rai, P.K. Rai, S. Bajaj, A. Kumar, Green Chem. 13, 1217 (2011)
V.K. Rai, R. Sharma, A. Kumar, Tetrahedron Lett. 54, 1071 (2013)
A. Kumar, S. Jamwal, S. Khan, N. Singh, V.K. Rai, Phosphorus Sulfur Silicon Relat. Elem. 192, 381 (2017)
Wayne, P.A.: Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically CLSI document M07-A8. Clinical and Laboratory Standards Institute (2008)
Wayne, P.A.: Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of yeasts, approved standard. CLSI document M27-A3. Clinical and Laboratory Standards Institute (2008)
Wayne, P.A.: Clinical and Laboratory Standards Institute. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi, approved standard. CLSI document M38-A2. Clinical and Laboratory Standards Institute (2008)
Acknowledgements
We are thankful to SAIF Chandigarh for providing spectral data. P. K. thanks to UGC, Govt. of India for BSR fellowship.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kour, P., Kumar, A., Sharma, R. et al. Synthesis of 2-amino-4H-chromen-4-ylphosphonates and β-phosphonomalonates via tandem Knoevenagel–Phospha-Michael reaction and antimicrobial evaluation of newly synthesized β-phosphonomalonates. Res Chem Intermed 43, 7319–7329 (2017). https://doi.org/10.1007/s11164-017-3077-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-017-3077-2