Abstract
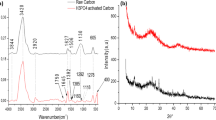
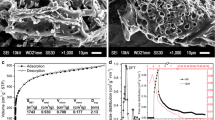
Activated carbon (AC) was prepared from sapelli wood sawdust using a microwave heating process. The biomass was mixed with inorganic components (lime + ZnCl2 and FeCl3) to form a homogeneous paste. The AC samples are denoted as AC-1A (100 g sapelli wood sawdust + 20 g lime + 80 g ZnCl2), AC-2A (150 g sapelli wood sawdust + 20 g lime + 80 g ZnCl2), AC-1B (100 g sapelli wood sawdust + 20 g lime + 40 g ZnCl2 + 40 g FeCl3), and AC-2B (150 g sapelli wood sawdust + 20 g lime + 40 g ZnCl2 + 40 g FeCl3). The samples were placed in a microwave oven and pyrolyzed under nitrogen flow. To increase their porosity, the pyrolyzed samples were subjected to a leaching process (with 6 mol L−1 HCl) under reflux to eliminate inorganic components. Several analytical techniques such as Fourier-transform infrared (FTIR) spectroscopy, scanning electron microscopy (SEM), and N2 isotherm and vapor adsorption analyses were performed to characterize the AC materials. The samples presented high Brunauer–Emmett–Teller (BET) surface areas, up to 941.08 m2 g−1 for AC-1A. The AC materials were tested for their o-cresol removal ability by determining the best fits to equilibrium and kinetic data using the Sips isotherm and fractional-order model, respectively. The maximum adsorption capacity of the AC samples as obtained from the Sips model was correlated with the surface area. The proposed adsorption mechanism suggests that hydrogen bonding, donor–acceptor complexation, and π–π interactions play key roles. The adsorbents were also tested for treatment of simulated industrial effluents, showing very good efficiency. Almost complete regeneration of the AC adsorbents was achieved using 10 % EtOH + 5 mol L−1 NaOH as eluent. These results demonstrate that sapelli wood sawdust is a promising precursor for preparation of AC to remove o-cresol from aqueous solution.








Similar content being viewed by others
References
R.G. Dolatto, I. Messerschmidt, B.F. Pereira, R. Martinazzo, G. Abate, Preconcentration of polar phenolic compounds from water samples and soil extract by liquid-phase microextraction and determination via liquid chromatography with ultraviolet detection. Talanta 148, 292–300 (2016)
H. Babich, D.L. Davis, Phenol: a review of environmental and health risks. Regul. Toxicol. Pharm. 1, 90–109 (1981)
C.A. Arenal, B.E. Sample, Wildlife toxicity assessment for phenol, in Wildlife toxicity assessments for chemicals of military concern, ed. by M.S. Johnson, M. Williams, G. Reddy, M. Quinn (Elsevier, Amsterdam, 2015), pp. 555–579
R. Wissiack, E. Rosenberg, Universal screening method for the determination of US Environmental Protection Agency phenols at the lower ng l−1 level in water samples by on-line solid-phase extraction-high-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry within a single run. J. Chromatogr. A 963, 149–157 (2002)
H. Pasdar, R. Marand, Effect of phenol loading on wastewater treatment by activated sludge process. J. Basic Appl. Sci. Res. 3, 121–126 (2013)
C.E. Paisio, M.R. Quevedo, M.A. Talano, P.S. González, E. Agostini, Application of two bacterial strains for wastewater bioremediation and assessment of phenolics biodegradation. Environ. Technol. 35, 1802–1810 (2014)
T. Saitoh, K. Fukushima, A. Miwa, Combined use of surfactant-induced coagulation of poly(allylamine hydrochloride) with peroxidase-mediated degradation for the rapid removal of estrogens and phenolic compounds from water. Sep. Purif. Technol. 128, 11–17 (2014)
F. Bazzarelli, E. Piacentini, T. Poerio, R. Mazzei, A. Cassano, L. Giorno, Advances in membrane operations for water purification and biophenols recovery/valorization from OMWWs. J. Membr. Sci. 497, 402–409 (2016)
M. Irani, L.R. Rad, H. Pourahmad, I. Haririan, Optimization of the combined adsorption/photo-Fenton method for the simultaneous removal of phenol and paracetamol in a binary system. Micropor. Mesopor. Mater. 206, 1–7 (2015)
P.V. Aken, R.V. den Broeck, J. Degrève, R. Dewil, The effect of ozonation on the toxicity and biodegradability of 2,4-dichlorophenol-containing wastewater. Chem. Eng. J. 280, 728–736 (2015)
A.M. Al-Hamdi, M. Sillanpaa, J. Dutta, Intermediate formation during photodegradation of phenol using lanthanum doped tin dioxide nanoparticles. Res. Chem. Intermed. 42, 3055–3069 (2016)
T.T.T. Dang, S.T.T. Le, D. Channei, W. Khanitchaidecha, A. Nakaruk, Photodegradation mechanisms of phenol in the photocatalytic process. Res. Chem. Intermed. (2016). doi:10.1007/s11164-015-2417-3
N. Douara, B. Bestani, N. Benderdouche, L. Duclaux, Sawdust-based activated carbon ability in the removal of phenol-based organics from aqueous media. Desalin. Water Treat. 57, 5529–5545 (2016)
N. Singh, C. Balomajumder, Simultaneous biosorption and bioaccumulation of phenol and cyanide using coconut shell activated carbon immobilized Pseudomonas putida (MTCC 1194). J. Environ. Chem. Eng. 4, 1604–1614 (2016)
J. Pal, M.K. Deb, D.K. Deshmukh, Removal of phenol in aqueous solution by adsorption onto green synthesized coinage nanoparticles beads. Res. Chem. Intermed. 41, 8363–8379 (2015)
R. Sudha, K. Srinivasan, P. Premkumar, Kinetic, mechanism and equilibrium studies on removal of Pb(II) using Citrus limettioides peel and seed carbon. Res. Chem. Intermed. 42, 1677–1697 (2016)
G.S. dos Reis, M. Wilhelm, T.C.A. Silva, K. Rezwan, C.H. Sampaio, E.C. Lima, S.M.A.G.U. Souza, The use of design of experiments for the evaluation of the production of surface-rich activated carbon from sewage sludge via microwave and conventional pyrolysis. Appl. Therm. Eng. 93, 590–597 (2016)
G.S. dos Reis, M.A. Adebayo, E.C. Lima, C.H. Sampaio, L.D.T. Prola, Activated carbon from sewage sludge for preconcentration of copper. Anal. Lett. 49, 541–555 (2016)
P. Humpola, H. Odetti, J.C. Moreno-Pirajan, L. Giraldo, Activated carbons obtained from agro-industrial waste: textural analysis and adsorption environmental pollutants. Adsorption 22, 23–31 (2016)
B. Acevedo, R.P. Rocha, M.F.R. Pereira, J.L. Figueiredo, C. Barriocanal, Adsorption of dyes by ACs prepared from waste tyre reinforcing fibre: effect of texture, surface chemistry and pH. J. Colloid Interf. Sci. 459, 189–198 (2015)
S. Malathi, N. Krishnaveni, R. Sudha, Adsorptive removal of lead(II) from an aqueous solution by chemically modified cotton seed cake. Res. Chem. Intermed. 42, 2285–2302 (2016)
M.J. Ahmed, Application of agricultural based activated carbons by microwave and conventional activations for basic dye adsorption: review. J. Environ. Chem. Eng. 4, 89–99 (2016)
Anonyme, Etude sur la traçabilité des bois exploités au Cameroun et des produits bois » exportés à partir du Cameroun [Study on the traceability of timber exploited in Cameroon and products “Wood” exported from Cameroon], Ministère des Forêts et de la Faune du Cameroun-Rapport de Tecsult Interna-tional Lte é, Yaounde (2007)
J.M. Sieliechi, P.S. Thue, Removal of paraquat from drinking water by activated carbon prepared from waste wood. Desalin. Water Treat. 55, 986–998 (2015)
R.H. Hesas, A. Arami-Niya, W.M.A.W. Daud, J.N. Sahu, Comparison of oil palm shell-based activated carbons produced by microwave and conventional heating methods using zinc chloride activation. J. Anal. Appl. Pyrolysis 104, 176–184 (2013)
X. Ge, X. Ma, Z. Wu, X. Xiao, Y. Yan, Modification of coal-based activated carbon with nitric acid using microwave radiation for adsorption of phenanthrene and naphthalene. Res. Chem. Intermed. 41, 7327–7347 (2015)
C. Saucier, M.A. Adebayo, E.C. Lima, R. Cataluna, P.S. Thuea, L.D.T. Prola, M.J. Puchana-Rosero, F.M. Machado, F.A. Pavan, G.L. Dotto, Microwave-assisted activated carbon from cocoa shell as adsorbent for removal of sodium diclofenac and nimesulide from aqueous effluents. J. Hazard. Mater. 289, 18–27 (2015)
C. Saucier, M.A. Adebayo, E.C. Lima, L.D.T. Prola, P.S. Thue, C.S. Umpierres, M.J. Puchana-Rosero, F.M. Machado, Comparison of a homemade Bacury shell activated carbon with MWCNT for the removal of Brilliant Blue FCF food dye from aqueous solutions. Clean: Air, Soil, Water 43, 1389–1400 (2015)
J.C.P. Vaghetti, M. Zat, K.R.S. Bentes, L.S. Ferreira, E.V. Benvenutti, E.C. Lima, 4-Phenylenediaminepropylsilica xerogel as a sorbent for copper determination in waters by slurry-sampling ETAAS. J. Anal. At. Spectrom. 18, 376–380 (2003)
L.D.T. Prola, E. Acayanka, E.C. Lima, C.S. Umpierres, J.C.P. Vaghetti, W.O. Santos, S. Laminsi, P.T. Njifon, Comparison of Jatropha curcas shells in natural form and treated by non-thermal plasma as biosorbents for removal of reactive red 120 textile dye from aqueous solution. Ind. Crop. Prod. 46, 328–340 (2013)
S.L. Goertzen, K. Theriault, A.M. Oickle, A.C. Tarasuk, H.A. Andreas, Standardization of the Boehm titration: Part I—CO2 expulsion and endpoint determination. Carbon 48, 1252–1261 (2010)
E.C. Lima, R.V. Barbosa, J.L. Brasil, A.H.D.P. Santos, Evaluation of different permanent modifiers for the determination of arsenic, cadmium and lead in environmental samples by electrothermal atomic absorption spectrometry. J. Anal. At. Spectrom. 17, 1523–1529 (2002)
E.C. Lima, F. Barbosa Jr., F.J. Krug, U. Guaita, Tungsten–rhodium permanent chemical modifier for lead determination in digests of biological materials and sediments by electrothermal atomic absorption spectrometry. J. Anal. At. Spectrom. 14, 1601–1605 (1999)
E.C. Lima, F.J. Krug, J.A. Nóbrega, A.R.A. Nogueira, Determination of ytterbium in animal faeces by tungsten coil electrothermal atomic absorption spectrometry. Talanta 47, 613–623 (1998)
E.C. Lima, P.G. Fenga, J.R. Romero, W.F. de Giovani, Electrochemical behaviour of [Ru(4,4′-Me2bpy)2(PPh3)(H2O)](ClO4)2 in homogeneous solution and incorporated into carbon paste electrodes: application to oxidation of benzylic compounds. Polyhedron 17, 313–318 (1998)
W.S. Alencar, E.C. Lima, B. Royer, B.D. dos Santos, T. Calvete, E.A. da Silva, C.N. Alves, Application of aqai stalks as biosorbents for the removal of the dye Procion Blue MX-R from aqueous solution. Sep. Sci. Technol. 47, 513–526 (2012)
E.C. Lima, M.A. Adebayo, F.M. Machado, Chapter 3-kinetic and equilibrium models of adsorption, in Carbon nanomaterials as adsorbents for environmental and biological applications, ed. by C.P. Bergmann, F.M. Machado (Springer, Berlin, 2015), pp. 33–69
K.S.W. Sing, D.H. Everett, R.A.W. Haul, L. Moscou, R.A. Pierotti, J. Rouquerol, T. Siemieniewska, Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (recommendations 1984). Pure Appl. Chem. 57, 603–619 (1985)
O. Pezoti Jr., A.L. Cazetta, I.P.A.F. Souza, K.C. Bedin, A.C. Martins, T.L. Silva, V.C. Almeida, Adsorption studies of methylene blue onto ZnCl2-activated carbon produced from buriti shells (Mauritia flexuosa L.). J. Ind. Eng. Chem. 20, 4401–4407 (2014)
R.G. Pereira, C.M. Veloso, N.M. da Silva, L.F. de Sousa, R.C.F. Bonomo, A.O. de Souza, M.O.G. Souza, R.C.I. Fontan, Preparation of activated carbons from cocoa shells and siriguela seeds using H3PO4 and ZnCl2 as activating agents for BSA and α-lactalbumin adsorption. Fuel Proc. Technol. 126, 476–486 (2014)
O. Pezoti Jr., A.L. Cazetta, R.C. Gomes, E.O. Barizão, I.P.A.F. Souza, A.C. Martins, T. Asefa, V.C. Almeida, Synthesis of ZnCl2-activated carbon from macadamia nut endocarp (Macadamia integrifolia) by microwave-assisted pyrolysis: optimization using RSM and methylene blue adsorption. J. Anal. Appl. Pyrol. 105, 166–176 (2014)
T.-H. Liou, Development of mesoporous structure and high adsorption capacity of biomass-based activated carbon by phosphoric acid and zinc chloride activation. Chem. Eng. J. 158, 129–142 (2010)
Z. Hu, M.P. Srinivasan, Mesoporous high surface area activated carbon. Micropor. Mesopor. Mater. 43, 267–275 (2001)
A.C. Lua, T. Yang, Characteristics of activated carbon prepared from pistachionut shell by zinc chloride activation under nitrogen and vacuum conditions. J. Colloid Interface Sci. 290, 505–513 (2005)
Z. Hu, M.P. Srinivasan, Y. Ni, Novel activation process for preparing highly microporous and mesoporous activated carbons. Carbon 39, 877–886 (2001)
V. Gomez-Serrano, E.M. Cuerda-Correa, M.C. Fernandez-Gonzales, M.F. Alexandre-Franco, A. Macıas-Garcıa, Preparation of activated carbons from chestnut wood by phosphoric acid-chemical activation: study of microporosity and fractal dimension. Mater. Lett. 59, 846–853 (2005)
T. Calvete, E.C. Lima, N.F. Cardoso, S.L.P. Dias, E.S. Ribeiro, Removal of brilliant green dye from aqueous solutions using home-made activated carbons. Clean: Air, Soil, Water 38, 521–532 (2010)
G.S. dos Reis, C.H. Sampaio, E.C. Lima, M. Wilhelm, Preparation of novel adsorbents based on combinations of polysiloxanes and sewage sludge to remove pharmaceuticals from aqueous solutions. Colloids Surf. A 497, 304–315 (2016)
M. Wilhelm, C. Soltmann, D. Koch, G. Grathwohl, Ceramers: functional materials for adsorption techniques. J. Eur. Ceram. Soc. 25, 271–276 (2005)
T. Prenzel, M. Wilhelm, K. Rezwan, Tailoring amine functionalized hybrid ceramics to control CO2 adsorption. Chem. Eng. J. 235, 198–206 (2014)
M.J. Puchana-Rosero, M.A. Adebayo, E.C. Lima, F.M. Machado, P.S. Thue, J.C.P. Vaghetti, C.S. Umpierres, M. Gutterres, Microwave-assisted activated carbon obtained from the sludge of tannery-treatment effluent plant for removal of leather dyes. Colloids Surf. A 504, 105–115 (2016)
F.M. Machado, C.P. Bergmann, E.C. Lima, B. Royer, F.E. de Souza, I.M. Jauris, T. Calvete, S.B. Fagan, Adsorption of reactive blue 4 dye from water solutions by carbon nanotubes: experiment and theory. Phys. Chem. Chem. Phys. 14, 11139–11153 (2012)
N.F. Cardoso, E.C. Lima, B. Royer, M.V. Bach, G.L. Dotto, L.A.A. Pinto, T. Calvete, Comparison of Spirulina platensis microalgae and commercial activated carbon as adsorbents for the removal of reactive red 120 dye from aqueous effluents. J. Hazard. Mater. 241–242, 146–153 (2012)
G.L. Dotto, E.C. Lima, L.A.A. Pinto, Biosorption of food dyes onto Spirulina platensis nanoparticles: equilibrium isotherm and thermodynamic analysis. Bioresour. Technol. 103, 123–130 (2012)
A. Dabrowski, Adsorption: from theory to practice. Adv. Colloid Interface Sci. 93, 135–224 (2001)
O. Hamdaoui, E. Naffrechoux, L. Petrier, C. Tifouti, Effects of ultrasound on adsorption–desorption of p-chlorophenol on granular activated carbon. Ultrason. Sonochem. 10, 109–114 (2003)
H.-M. Shen, G.-Y. Zhu, W.-B. Yu, H.-K. Wu, H.-B. Ji, H.-X. Shi, Y.-B. She, Y.-F. Zheng, Fast adsorption of p-nitrophenol from aqueous solution using cyclodextrin grafted silica gel. Appl. Surf. Sci. 356, 1155–1167 (2015)
S. Suresh, V.C. Srivastava, I.M. Mishra, Study of catechol and resorcinol adsorption mechanism through granular activated carbon: characterization, pH and kinetic study. Sep. Sci. Technol. 46, 1750–1766 (2011)
Z. Rawajfih, N. Nsour, Sorption of phenol and 4-chlorophenol onto pumice treated with cationic surfactant. J. Colloid Interface Sci. 298, 39–49 (2006)
Q.-S. Liu, T. Zheng, P. Wang, J.-P. Jiang, N. Li, Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem. Eng. J. 157, 348–356 (2010)
Acknowledgments
We would like to thank the National Council for Scientific and Technological Development (CNPq, Brazil) and The Academy of Sciences for Developing World (TWAS, Italy) for financial support and sponsorship. We also thank The Centre of Electron Microscopy (CME-UFRGS) for use of the SEM. We are once again grateful to Chemaxon for providing us with an academic research license for Marvin Sketch software version 16.6.6.0 (http://www.chemaxon.com) 2016, used for o-cresol physical–chemical properties.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Thue, P.S., dos Reis, G.S., Lima, E.C. et al. Activated carbon obtained from sapelli wood sawdust by microwave heating for o-cresol adsorption. Res Chem Intermed 43, 1063–1087 (2017). https://doi.org/10.1007/s11164-016-2683-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-016-2683-8