Abstract
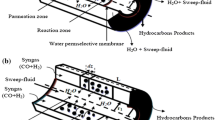
In this work, the performance of the Fischer–Tropsch reaction over iron catalyst in three reactor configurations was theoretically studied and analyzed. Indeed, the control and the guidance of produced hydrocarbons composition were done using a new concept of membrane separation reactor. Our findings show that the composition can be imposed by the nature of the integrated membrane. Depending on the used membrane, the water gas shift reaction equilibrium can be shifted towards hydrogen production or towards carbon monoxide production and consequently, the H2/CO ratio was changed in situ for giving olefins or paraffin according to our requirements specification. Finally, it was found that using a water permselective membrane can boost the composition to C3–C5 olefin compounds, whereas the separation of carbon dioxide can enhance the formation of paraffins.



Similar content being viewed by others
Abbreviations
- \(A\) :
-
Cross-section (\({\text{m}}^{2}\))
- \(C_{p}\) :
-
Specific heat transfer at constant pressure (\({\text{J mol}}^{ - 1} {\text{K}}^{ - 1}\))
- \(C_{pg}\) :
-
Specific heat transfer of gaseous mixture at constant pressure (\({\text{J mol}}^{ - 1} {\text{K}}^{ - 1}\))
- \(d_{p}\) :
-
Diameter of catalyst particle (\({\text{m}}\))
- \(D_{C}\) :
-
Diameter of cooling tube (\({\text{m}}\))
- Ð(θξ) :
-
Diffusivity of component \(\xi\) (\({\text{m}}^{2} {\text{s}}^{ - 1}\))
- Ð0,ξ :
-
Diffusivity of component \(\xi\) at zero loadings and infinite temperature (\({\text{m}}^{2} {\text{s}}^{ - 1}\))
- Ðθξ=0 :
-
Diffusivity of component \(\xi\) at zero loadings (\({\text{m}}^{2} {\text{s}}^{ - 1}\))
- \(E_{5}\) :
-
Activation energy for paraffin formation (\({\text{J mol}}^{ - 1}\))
- \(E_{5,M}\) :
-
Activation energy for methane formation (\({\text{J mol}}^{ - 1}\))
- \(E_{6}\) :
-
Activation energy for olefin formation (\({\text{J mol}}^{ - 1}\))
- \(E_{v}\) :
-
Activation energy for WGS reaction (\({\text{J mol}}^{ - 1}\))
- \(E_{dif,\xi }\) :
-
Diffusivity activation energy of component \(\xi\) (\({\text{J mol}}^{ - 1}\))
- \(F_{i}\) :
-
Molar flow rate of hydrocarbon \(i\) (\({\text{mol s}}^{ - 1}\))
- \(F_{T}\) :
-
Total molar flow rate (\({\text{mol s}}^{ - 1}\))
- \(F_{T}^{0}\) :
-
Initial molar flow rate (\({\text{mol s}}^{ - 1}\))
- \(F_{IN}\) :
-
Molar flow rate of inert gases (\({\text{mol s}}^{ - 1}\))
- \(GHSV\) :
-
Gas hourly space velocity (\({\text{h}}^{ - 1}\))
- \({\text{I}}_{{{\text{index}}}}\) :
-
Fraction of inert gas
- \(J_{\xi }\) :
-
Permeation flux of component \(\xi\) (\({\text{mol m}}^{ - 2} {\text{s}}^{ - 1}\))
- \(k_{1}\) :
-
Rate constant of CO adsorption (\({\text{mol kg}}^{ - 1} {\text{s}}^{ - 1} {\text{bar}}^{ - 1}\))
- \(k_{5}\) :
-
Rate constant of paraffin formation (\({\text{mol kg}}^{ - 1} {\text{s}}^{ - 1} {\text{bar}}^{ - 1}\))
- \(k_{5,0}\) :
-
Preexponenetial factor of rate constant of paffin formation (\({\text{mol kg}}^{ - 1} {\text{s}}^{ - 1} {\text{bar}}^{ - 1}\))
- \(k_{5M}\) :
-
Rate constant of methane formation (\({\text{mol kg}}^{ - 1} {\text{s}}^{ - 1} {\text{bar}}^{ - 1}\))
- \(k_{5M,0}\) :
-
Pre-exponential factor of rate constant of methane formation (\({\text{mol kg}}^{ - 1} {\text{s}}^{ - 1} {\text{bar}}^{ - 1}\))
- \(k_{6}\) :
-
Rate constant of olefin desorption reaction (\({\text{mol kg}}^{ - 1} {\text{s}}^{ - 1}\))
- \(k_{6,0}\) :
-
Pre-exponential factor of rate constant of olefin desorption reaction (\({\text{mol kg}}^{ - 1} {\text{s}}^{ - 1}\))
- \(k_{ - 6}\) :
-
Rate constant of olefin re-adsorption reaction (\({\text{mol kg}}^{ - 1} {\text{s}}^{ - 1} {\text{bar}}^{ - 1}\))
- \(k_{v}\) :
-
Rate constant of \({\text{CO}}_{2}\) formation (\({\text{mol kg}}^{ - 1} {\text{s}}^{ - 1} {\text{bar}}^{ - 1.5}\))
- \(k_{v,0}\) :
-
Pre-exponential factor of rate constant of \({\text{CO}}_{2}\) formation (\({\text{mol kg}}^{ - 1} {\text{s}}^{ - 1} {\text{bar}}^{ - 1.5}\))
- \(K_{2}\) :
-
Equilibrium constant of \({\text{CH}}\) intermediate formation
- \(K_{3}\) :
-
Equilibrium constant of \({\text{CH}}_{2}\) intermediate formation
- \(K_{4}\) :
-
Equilibrium constant of \({\text{CH}}_{3}\) alkyl formation
- \(K_{v}\) :
-
Group of constants in WGS reaction
- \(K_{\xi }\) :
-
Adsorption equilibrium constant of component \(\xi\) (\({\text{bar}}^{ - 1}\))
- \(K_{\xi ,0}\) :
-
Adsorption equilibrium constant of component \(\xi\) at infinite temperature (\({\text{bar}}^{ - 1}\))
- \(K_{WGS}\) :
-
Equilibrium constant of WGS reaction
- \(L\) :
-
Reactor length (\({\text{m}}\))
- \(l\) :
-
Dimensionless reactor length
- \(M\) :
-
Inlet molar flow ratio between hydrogen and carbon monoxide
- \(O/P\) :
-
Olefin over paraffin selectivity ratio
- \(P_{i}\) :
-
Partial pressure of hydrocarbon \(i\) (\({\text{bar}}\))
- \(P_{IN}\) :
-
Partial pressure of inert gas (\({\text{bar}}\))
- \(P_{per}\) :
-
Partial pressure in permeate side (\({\text{bar}}\))
- \(P_{per,tot}\) :
-
Total pressure in permeate side (\({\text{bar}}\))
- \(P_{rec}\) :
-
Partial pressure in reaction side (\({\text{bar}}\))
- \(P_{T}\) :
-
Total pressure in reaction side (\({\text{bar}}\))
- \(P_{T}^{0}\) :
-
Initial total pressure in reaction side (\({\text{bar}}\))
- \(P_{\xi }\) :
-
Partial pressure of component \(\xi\) (\({\text{bar}}\))
- \(q_{\xi }\) :
-
Amount adsorbed of component \(\xi \left( {{\text{mol kg}}^{ - 1} } \right)\)
- \(q_{\xi }^{sat}\) :
-
Saturation amount adsorbed of component \(\xi \left( {{\text{mol kg}}^{ - 1} } \right)\)
- \(R\) :
-
Universal gas constant (8.314 \({\text{J mol}}^{ - 1} {\text{K}}^{ - 1}\))
- \(R_{j}\) :
-
Rate of reaction \(j\) (\({\text{mol kg}}^{ - 1} {\text{s}}^{ - 1}\))
- \(R_{{C_{n} H_{2n + 2} }}\) :
-
Paraffin reaction rate (\({\text{mol kg}}^{ - 1} {\text{s}}^{ - 1}\))
- \(R_{{C_{n} H_{2n} }}\) :
-
Olefin reaction rate (\({\text{mol kg}}^{ - 1} {\text{s}}^{ - 1}\))
- \(R_{WGS}\) :
-
Water–gas shift reaction rate (\({\text{mol kg}}^{ - 1} {\text{s}}^{ - 1}\))
- \(S_{i}\) :
-
Hydrocarbonsselectivity (\(\%\))
- \(T\) :
-
Temperature (\({\text{K}}\))
- \(T_{sh}\) :
-
Shell temperature (\({\text{K}}\))
- \(U_{sh}\) :
-
Heat transfer coefficient shell-gases (\({\text{W m}}^{ - 2} {\text{ K}}^{ - 1}\))
- \(\nu\) :
-
Gas linear velocity (\({\text{m s}}^{ - 1}\))
- \(x\) :
-
Membrane coordinate
- \(y\) :
-
Molar fraction
- \(z\) :
-
Axial reactor coordinate
- \(\varepsilon\) :
-
Porosity of catalytic bed
- \(\varepsilon_{m}\) :
-
Porosity of membrane-support layer
- \(\rho\) :
-
Catalyst density(\({\text{kg m}}^{ - 3}\))
- \(\rho_{g}\) :
-
Gas density (\({\text{kg m}}^{ - 3}\))
- \(\rho_{m}\) :
-
Membrane density (\({\text{kg m}}^{ - 3}\))
- \(\upsilon_{ij}\) :
-
Stoichiometric coefficient of hydrocarbon \(i\) in reaction \(j\)
- \(\mu\) :
-
Gas dynamic viscosity (\({\text{bar s}}^{ - 1}\))
- \(\delta\) :
-
Membrane thickness (\({\text{m}}\))
- \(\theta_{\xi }\) :
-
Fractional sites occupancy for component \(\xi\)
- \(\Delta H_{{R_{j} }}\) :
-
Enthalpy of reaction \(j\) (\({\text{J mol}}^{ - 1}\))
- \(\Delta H_{ads, {\xi}}\) :
-
Adsorption enthalpy of component \(\xi\) (\({\text{J mol}}^{ - 1}\))
- \(g\) :
-
Gas-phase
- \(i\) :
-
Index indicating hydrocarbons
- \(IN\) :
-
Inert gases
- \(j\) :
-
Index indicating reactions
- \(m\) :
-
Membrane
- \(n\) :
-
Chain length of hydrocarbons
- \(0\) :
-
Inlet reactor
- \(BTL\) :
-
Biomass to liquid
- \(CTL\) :
-
Coal to liquid
- \(CR\) :
-
Conventional reactor
- \(FT\) :
-
Fischer–Tropsch
- \(GTL\) :
-
Gas To liquid
- \(MRC\) :
-
Membrane reactor for carbon dioxide removal
- \(MRW\) :
-
Membrane reactor for water removal
- \(WGS\) :
-
Water–gas-shift
References
Karimipourfard D, Nemati N, Bahrani S, Rahimpour MR (2018) Simultaneous increase of H2 and gasoline production by optimizing thermally coupled methanol steam reforming with Fischer–Tropsch synthesis. Chem Prod Process Model 13:14
Nazir MS, Mahdi AJ, Bilal M et al (2019) Environmental impact and pollution-related challenges of renewable wind energy paradigm—a review. Sci Total Environ 683:436–444
Sullivan TJ, Driscoll CT, Beier CM et al (2018) Air pollution success stories in the United States: the value of long-term observations. Environ Sci Policy 84:69–73
Moazami N, Mahmoudi H, Panahifar P et al (2015) Mathematical Modeling and Performance Study of Fischer–Tropsch synthesis of liquid fuel over cobalt-silica. Energy Proced 75:62–71
Haghtalab A, Shariati J, Mosayebi A (2019) Experimental and kinetic modeling of Fischer–Tropsch synthesis over nano structure catalyst of Co–Ru/carbon nanotube. React Kinet Mech Catal 126:1003–1026
Martínez del Monte D, Vizcaíno AJ, Dufour J, Martos C (2019) Effect of K, Co and Mo addition in Fe-based catalysts for aviation biofuels production by Fischer–Tropsch synthesis. Fuel Process Technol 194:106102
Janani H, Mirzaei AA, Rezvani A (2019) Correlation of metal–organic framework structures and catalytic performance in Fischer–Tropsch synthesis process. React Kinet Mech Catal 128:205–215
Donnelly TJ, Satterfield CN (1989) Product distributions of the Fischer–Tropsch synthesis on precipitated iron catalysts. Appl Catal 52:93–114
Alihellal D, Chibane L (2016) Simulation study of the effect of water removal from Fischer–Tropsch products on the process performance using a hydrophilic membrane reactor. React Kinet Mech Catal 117:605–621
Rohde MP, Unruh D, Schaub G (2005) Membrane application in Fischer−Tropsch synthesis to enhance CO2 hydrogenation. Ind Eng Chem Res 44:9653–9658
Rohde MP (2011) In-situ H2O removal via hydrophilic membranes during Fischer–Tropsch and other fuel-related synthesis reactions. KIT Scientific Publishing, Karlsruhe
Espinoza RL, du Toit E, Santamaria J et al (2000) Use of membranes in Fischer–Tropsch reactors. Stud Surf Sci Catal 130:389–394
Yang JZ, Liu QL, Wang HT (2007) Analyzing adsorption and diffusion behaviors of ethanol/water through silicalite membranes by molecular simulation. J Membr Sci 291:1–9
Zhu W, Gora L, van den Berg AWC et al (2005) Water vapour separation from permanent gases by a zeolite-4A membrane. J Membr Sci 253:57–66
Pera-Titus M, Fité C, Sebastián V et al (2008) Modeling pervaporation of ethanol/water mixtures within ‘‘Real’’ Zeolite NaA membranes. Ind Eng Chem Res 47:3213–3224
Zhu W, Hrabanek P, Gora L et al (2006) Role of adsorption in the permeation of CH4 and CO2 through a silicalite-1 membrane. Ind Eng Chem Res 45:767–776
Rui Z, Ji H, Lin YS (2011) Modeling and analysis of ceramic–carbonate dual-phase membrane reactor for carbon dioxide reforming with methane. Int J Hydrog Energy 36:8292–8300
Algieri C, Bernardo P, Golemme G et al (2003) Permeation properties of a thin silicalite-1 (MFI) membrane. J Membr Sci 222:181–190
Guo S, Yu C, Gu X et al (2011) Simulation of adsorption, diffusion, and permeability of water and ethanol in NaA zeolite membranes. J Membr Sci 376:40–49
Liu D, Zhang Y, Jiang J et al (2015) High-performance NaA zeolite membranes supported on four-channel ceramic hollow fibers for ethanol dehydration. RSC Adv 5:95866–95871
Bakker WJW, Van Den Broeke LJP, Kapteijn F, Moulijn JA (1997) Temperature dependence of one-component permeation through a silicalite-1 membrane. AIChE J 43:2203–2214
Wang Y-N, Ma W-P, Lu Y-J et al (2003) Kinetics modelling of Fischer–Tropsch synthesis over an industrial Fe–Cu–K catalyst. Fuel 82:195–213
Kwack S-H, Park M-J, Bae JW et al (2011) Development of a kinetic model of the Fischer–Tropsch synthesis reaction with a cobalt-based catalyst. React Kinet Mech Catal 104:483–502
Rahimpour MR, Elekaei H (2009) Optimization of a novel combination of fixed and fluidized-bed hydrogen-permselective membrane reactors for Fischer–Tropsch synthesis in GTL technology. Chem Eng J 152:543–555
Poshusta JC, Noble RD, Falconer JL (2001) Characterization of SAPO-34 membranes by water adsorption. J Membr Sci 186:25–40
Lovallo MC, Gouzinis A, Tsapatsis M (1998) Synthesis and characterization of oriented MFI membranes prepared by secondary growth. AIChE J 44:1903–1913
Burggraaf AJ, Vroon ZAEP, Keizer K, Verweij H (1998) Permeation of single gases in thin zeolite MFI membranes. J Membr Sci 144:77–86
Poshusta JC, Noble RD, Falconer JL (1999) Temperature and pressure effects on CO2 and CH4 permeation through MFI zeolite membranes. J Membr Sci 160:115–125
van den Bergh J, Tihaya A, Kapteijn F (2010) High temperature permeation and separation characteristics of an all-silica DDR zeolite membrane. Microporous Mesoporous Mater 132:137–147
van den Bergh J, Zhu W, Groen JC et al (2007) Natural gas purification with a DDR zeolite membrane; permeation modelling with maxwell-stefan equations. Stud Surf Sci Catal 170:1021–1027
Nagumo R, Takaba H, Nakao S (2003) Prediction of ideal permeability of hydrocarbons through an MFI-type zeolite membrane by a combined method using molecular simulation techniques and permeation theory. J Phys Chem B 107:14422–14428
Díaz-Trujillo LA, Toledo-Chávez G, Jiménez-García G et al (2018) Modelling laboratory Fischer–Tropsch synthesis using cobalt catalysts. Int J Chem React Eng 16:11
Visconti CG, Tronconi E, Lietti L et al (2007) Development of a complete kinetic model for the Fischer–Tropsch synthesis over Co/Al2O3 catalysts. Chem Eng Sci 62:5338–5343
Zhuo M, Tan KF, Borgna A, Saeys M (2009) Density Functional Theory Study of the CO insertion mechanism for Fischer−Tropsch synthesis over Co catalysts. J Phys Chem C 113:8357–8365
Davis BH (2001) Fischer–Tropsch synthesis: current mechanism and futuristic needs. Fuel Process Technol 71:157–166
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bellal, A., Chibane, L. A new concept for control and orientation of the distribution of clean hydrocarbons produced by Fischer–Tropsch synthesis over an industrial iron catalyst. Reac Kinet Mech Cat 129, 725–742 (2020). https://doi.org/10.1007/s11144-020-01726-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-020-01726-7