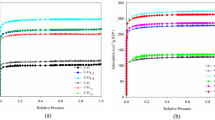
Abstract
Adsorbent materials based on apatites (non-substituted and substituted with Sr and Ba) were used for the adsorption of phenol from aqueous solution at 10, 20 and 30 °C and three pH values (3, 6 and 10.5). Structural studies show the formation of the apatite materials and the replacement of Ca by Sr and Ba. The adsorption isotherms of phenol were determined and modelled with two parameter equations (Langmuir, Freundlich and Temkin) or with three parameter equations (Sips and Dubinin–Radushkevich). Taking into account the Sips isotherm, the maximum amount of phenol uptake was 220 mg g−1 for Ba based adsorbent. Thermodynamic parameters were calculated and indicated that the adsorption of phenol onto prepared apatites was spontaneous and exothermic. The DRIFTS studies detected both phenol and phenolate species adsorbed on the surface. It was also shown that phenol molecules were adsorbed by hydrogen bonding between apatite metal ion or oxygen atom from P–O group and the hydrogen of the phenol functional group. The kinetic data indicate that physical and chemical adsorption, may be involved in the adsorption process.






Similar content being viewed by others
References
Dehghani MH, Mostofi M, Alimohammadi M, McKay G, Yetilmezsoy K, Albadarin AB, Heibati B, Al Ghouti M, Mubarak NM, Sahu JN (2016) High-performance removal of toxic phenol by single-walled and multi-walled carbon nanotubes: kinetics, adsorption, mechanism and optimization studies. J Ind Eng Chem 35:63–74
Victor-Ortega MD, Ochando-Pulido JM, Martinez-Ferez A (2016) Phenols removal from industrial effluents through novel polymeric resins: kinetics and equilibrium studies. Sep Purif Technol 160:136–144
Duarte-Davidson R, Troisi G, Capleton A (2016) A screening method for ranking chemicals by their fate and behaviour in the environment and potential toxic effects in human following non-occupational exposure. MRC Institute for Environment and Health, Leicester
D’Alessandro O, Thomas H, Sambeth JE (2014) Removal of phenol from aqueous solutions by adsorption onto Mn–Ce–K solids. Reac Kinet Mech Cat 113:257–267
Michalowicz J, Duda W (2007) Phenols—sources and toxicity. Polish J Environ Stud 16:347–362
Ontanon OM, Gonzales PS, Agostini E (2015) Biochemical and molecular mechanisms involved in simultaneous phenol and Cr(VI) removal by Acinetobacter guillouiae SFC 500-1A. Environ Sci Pollut Res 22:13014–13023
Fernandez MF, Arrebola JP, Jimenez-Diaza I, Saenz JM, Molina-Molina JM, Ballesteros O, Kortenkamp A, Olea N (2016) Bisphenol A and other phenols in human placenta from children with cryptorchidism or hypospadias. Reprod Toxicol 59:89–95
Jovic-Jovicic N, Mojovic Z, Darder M, Aranda P, Ruiz-Hitzky E, Bankovic P, Jovanovic D, Milutinovic-Nikolic A (2016) Smectite-chitosan-based electrodes in electrochemical detection of phenol and its derivatives. Appl Clay Sci 124–125:62–68
Hansch C, McCarns S, Smith C, Dodittle D (2000) Comparative QSAR evidence for a free-radical mechanism of phenol-induced toxicity. Chem Biol Interact 127:61–72
Divate SB, Hinge RV (2014) Review on research removal of phenol from wastewater by using different methods. Int J Sci Res Publ 4:1–3
Grabowska E, Reszczynska J, Zaleska A (2012) Mechanism of phenol photodegradation in the presence of pure and modified-TiO2: a review. Water Res 46:5453–5471
Wang Q, Li Y, Li J, Wang Y, Wang C, Wang P (2015) Experimental and kinetic study on the cometabolic biodegradation of phenol and 4-chlorophenol by psychrotrophic Pseudomonas putida LY1. Environ Sci Pollut Res 22:565–573
Ramavandi B, Jafarzadeh M, Sahebi S (2014) Removal of phenol from hyper-saline wastewater using Cu/Mg/Al–chitosan–H2O2 in a fluidized catalytic bed reactor. Reac Kinet Mech Cat 111:605–620
Daraei H, Mittal A, Noorisepehr M, Daraei F (2013) Kinetic and equilibrium studies of adsorptive removal of phenol onto eggshell waste. Environ Sci Pollut Res 20:4603–4611
Buema G, Cimpeanu SM, Sutiman D, Bucur RD, Rusu L, Cretescu I, Ciocinta RC, Harja M (2013) Lead removal from aqueous solution by bottom ash. J Food Agric Environ 11:1137–1141
Ciobanu G, Harja M, Diaconu M, Cimpeanu C, Teodorescu R, Bucur D (2014) Crystal violet dye removal from aqueous solution by nanohydroxyapatite. J Food Agric Environ 12:499–502
Gao X, Zhai X, Wang Z, Fu F, Li W (2015) Effective adsorption of phenol from aqueous solutions on activated semi-coke. J Mater Sci 50:4200–4208
Ma X, Zhang F, Wei L (2015) Effect of wood charcoal contents on the adsorption property, structure, and morphology of mesoporous activated carbon fibers derived from wood liquefaction process. J Mater Sci 50:1908–1914
Tseng RL, Wu KT, Wu FC, Juang RS (2010) Kinetic studies on the adsorption of phenol, 4-chlorophenol, and 2,4-dichlorophenol from water using activated carbons. J Environ Manage 91:2208–2214
Flores-Cano JV, Sanchez-Polo M, Messoud J, Velo-Gala I, Ocampo-Perez R, Rivera-Utrilla J (2016) Overall adsorption rate of metronidazole, dimetridazole and diatrizoate on activated carbons prepared from coffee residues and almond shells. J Environ Manage 169:116–125
Lin K, Pan J, Chen Y, Cheng R, Xu X (2009) Study the adsorption of phenol from aqueous solution on hydroxyapatite nanopowders. J Hazard Mater 161:231–240
Vila M, Sanchez-Salcedo S, Vallet-Regi M (2012) Hydroxyapatite foams for the immobilization of heavy metals: from waters to the human body. Inorg Chim Acta 393:24–35
Fierascu I, Fierascu RC, Popa O, Babeanu N (2014) Synthesized materials for decontamination of heavy metals polluted aqueous solutions. Rom Biotechnol Lett 19:9196–9202
Randorn C, Kanta A, Yaemsunthorn K, Rujijanakul G (2015) Fabrication of dense biocompatible hydroxyapatite ceramics with high hardness using a peroxide-based route: a potential process for scaling up. Ceram Int 41:5594–5599
Lin Y, Yang Z, Cheng J, Wang L (2008) Synthesis, characterization and antibacterial property of strontium half and totally substituted hydroxyapatite nanoparticles. J Wuhan Univ Technol 23:475–479
Fierascu I, Fierascu RC, Ion RM, Radovici C (2014) Synthesized apatitic materials for artefacts protection against biodeterioration. Rom J Mater 44:292–297
Dumitriu I, Fierascu RC, Bunghez IR, Ion RM (2009) Application of inductively coupled plasma—atomic emission spectroscopy (ICP-AES) based analysis for water quality control. Environ Eng Manag J 8:347–351
Mustafa S, Dilara B, Nargis K, Naeem A, Shahida P (2002) Surface properties of the mixed oxides of iron and silica. Colloids Surf A 205:273–282
Liao CJ, Lin FH, Chen KS, Sun JS (1999) Thermal decomposition and reconstitution of hydroxyapatite in air atmosphere. Biomaterials 20:1807–1813
Sofronia AM, Baies R, Anghel EM, Marinescu CA, Tanasescu S (2014) Thermal and structural characterization of synthetic and natural nanocrystalline hydroxyapatite. Mater Sci Eng C 43:153–163
Reza RA, Ahmed MJK, Sil AK, Ahmaruzzaman M (2014) A non-conventional adsorbent for the removal of clofibric acid from aqueous phase. Sep Sci Technol 49:1592–1603
Mekhamer WK, Al Andis N, El Shabanat M (2009) Kinetic study on the sedimentation behavior of Na- and Ca-kaolinite suspension in the presence of polyethyleneimine. J King Saud Univ Sci 21:125–132
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Ren R, Liu D, Li K, Sun J, Zhang C (2011) Adsorption of quaternary ammonium compounds onto activated sludge. J Water Resour Prot 3:105–113
Gunasekar V, Ponnusami V (2013) Kinetics, equilibrium and thermodynamic studies on adsorption of methylene blue by carbonized plant leaf powder. J Chem 2013:1–6
Desta MB (2013) Batch sorption experiments: Langmuir and Freundlich isotherm studies for the adsorption of textile metal ions onto Teff Straw (Eragrostis tef) agricultural waste. J Thermodyn 2013:1–6
Wei W, Yang L, Zhong WH, Li SY, Cui J, Wei ZG (2015) Fast removal of methylene blue from aqueous solution by adsorption onto poorly crystalline hydroxyapatite nanoparticles. Dig J Nanomater Biostruct 10:1343–1363
Abdelwahab O, Amin NK (2013) Adsorption of phenol from aqueous solutions by Luffa cylindrica fibers: kinetics, isotherm and thermodynamic studies. Egypt J Aquat Res 9:215–223
Milonjic SK (2007) A consideration of the correct calculation of thermodynamic parameters of adsorption. J Serb Chem Soc 72:1363–1367
Tahir SS, Rauf N (2003) Thermodynamic studies of Ni(II) adsorption onto bentonite from aqueous solution. J Chem Thermodyn 35:2003–2009
Sheng GD, Shao DD, Ren XM, Wang XQ, Li JX, Chen YX, Wang XK (2010) Kinetics and thermodynamics of adsorption of ionizable aromatic compounds from aqueous solutions by as-prepared and oxidized multiwalled carbon nanotubes. J Hazard Mater 178:505–516
Giraldo L, Moreno-Pirajan JC (2014) Study of adsorption of phenol on activated carbons obtained from eggshells. J Anal Appl Pyrolys 106:41–47
Tang W, Huang H, Gao Y, Liu X, Yang X, Ni H, Zhang J (2015) Preparation of a novel porous adsorption material from coal slag and its adsorption properties of phenol from aqueous solution. Mater Design 88:1191–1200
Boyd AR, Rutledge L, Randolph LD, Meenan BJ (2015) Strontium-substituted hydroxyapatite coatings deposited via a co-deposition sputter technique. Mater Sci Eng C 46:290–300
Boyd AR, O’Kane C, Meenan BJ (2013) Control of calcium phosphate thin film stoichiometry using multi-target sputter deposition. Surf Coat Technol 233:131–139
Gibson IR, Bonfield W (2002) Novel synthesis and characterization of an AB-type carbonate-substituted hydroxyapatite. J Biomed Mater Res 59:697–708
Kavitha M, Subramanian R, Narayanan R, Udhayabanu V (2014) Solution combustion synthesis and characterization of strontium substituted hydroxyapatite nanocrystals. Powder Technol 253:129–137
Lorenc-Grabowska E, Diez MA, Gryglewicz G (2016) Influence of pore size distribution on the adsorption of phenol on PET-based activated carbons. J Colloid Interface Sci 469:205–212
Nath K, Panchani S, Bhakhar MS, Chatrola S (2013) Preparation of activated carbon from dried pods of Prosopis cineraria with zinc chloride activation for the removal of phenol. Environ Sci Pollut Res Int 20:4030–4045
Gundogdu A, Duran C, Senturk HB, Soylak M, Ozdes D, Serencam H, Imamoglu M (2012) Adsorption of phenol from aqueous solution on a low-cost activated carbon produced from tea industry waste: equilibrium, kinetic, and thermodynamic study. J Chem Eng Data 57:2733–2743
Liu Q-S, Zheng T, Wang P, Jiang J-P, Li N (2010) Adsorption isotherm, kinetic and mechanism studies of some substituted phenols on activated carbon fibers. Chem Eng J 157:348–356
Singh KP, Malik A, Sinha S, Ojha P (2008) Liquid-phase adsorption of phenols using activated carbons derived from agricultural waste material. J Hazard Mater 150:626–641
Hameed BH, Rahman AA (2008) Removal of phenol from aqueous solutions by adsorption onto activated carbon prepared from biomass material. J Hazard Mater 160:576–581
Arana Rodriguez JM, Mazzoco RR (2010) Adsorption studies of methylene blue and phenol onto black stone cherries prepared by chemical activation. J Hazard Mater 180:656–661
Hamdaoui O, Naffrechoux E (2007) Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon. Part I. Two-parameter models and equations allowing determination of thermodynamic parameters. J Hazard Mater 147:381–394
Mohanty K, Das D, Biswas MN (2005) Adsorption of phenol from aqueous solutions using activated carbons prepared from Tectona grandis sawdust by ZnCl2 activation. Chem Eng J 115:121–131
Dursun AY, Kalayci CS (2005) Equilibrium, kinetic and thermodynamic studies on the adsorption of phenol onto chitin. J Hazard Mater 123:151–157
Acknowledgements
The present study was partially supported by a grant of the Romanian National Authority for Scientific Research and Innovation, CNCS/CCCDI–UEFISCDI, project number PN-III-P2-2.1-PED-2016-0198 and by a grant of the Romanian National Authority for Scientific Research and Innovation, CNCS/CCCDI–UEFISCDI, project number PN-III-P2-2.1-PED-2016-0251, within PNCDI III.
Author information
Authors and Affiliations
Contributions
I. Fierascu, S.M. Avramescu and R.C. Fierascu are the principal investigators in this study, having an equal contribution to the manuscript. IF, SMA and RCF conceived and designed the study, analysed and correlated the data. IF and RCF performed the synthesis and characterisation studies trough ICP-AES, XRD and EDXRF. SMA performed the phenol adsorption studies and FTIR analyses. I. Petreanu performed the thermal analyses, A. Marinoiu performed the BET analyses, A. Soare performed the SEM analyses and A. Nica contributed to the phenol adsorption studies. All authors read, contributed to and approved the final manuscript.
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Fierascu, I., Avramescu, S.M., Petreanu, I. et al. Efficient removal of phenol from aqueous solutions using hydroxyapatite and substituted hydroxyapatites. Reac Kinet Mech Cat 122, 155–175 (2017). https://doi.org/10.1007/s11144-017-1197-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11144-017-1197-8