Abstract
Purpose
Eastern Cooperative Oncology Group Performance Status (ECOG-PS) is currently an important parameter in the choice of treatment strategy for metastatic pancreatic adenocarcinoma (mPA) patients. However, previous research has shown that patients’ self-reported health-related quality of life (HRQOL) scales provided additional prognostic information in homogeneous groups of patients with respect to ECOG-PS. The aim of this study was to identify HRQOL scales with independent prognostic value in mPA and to propose prognostic groups for these patients.
Methods
We analysed data from 98 chemotherapy-naive patients with histologically proven mPA recruited from 2007 to 2011 in the FIRGEM phase II study which aimed to compare the effectiveness of two chemotherapy regimen. HRQOL data were assessed with the European Organization for Research and Treatment of Cancer QLQ-C30 questionnaire. A random survival forest methodology was used to impute missing data and to identify major prognostic factors for overall survival.
Results
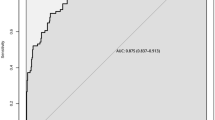
Baseline HRQOL assessment was completed by 60 % of patients (59/98). Twelve prognostic variables were identified. The three most important prognostic variables were fatigue, appetite loss, and role functioning, followed by three laboratory variables. The model’s discriminative power assessed by Harrell’s C statistic was 0.65. Fatigue score explained almost all the survival variability.
Conclusion
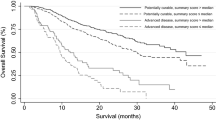
HRQOL scores have prognostic value for mPA patients with good ECOG-PS. Moreover, the patient’s fatigue, appetite loss, and self-perception of daily activities were more reliable prognostic indicators than clinical and laboratory variables. These HRQOL scores, especially the fatigue symptom, should be urgently included for prognostic assessment of mPA patients (with good ECOG-PS).





Similar content being viewed by others
References
Ferlay, J., Soerjomataram, I., Dikshit, R., et al. (2015). Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer, 136(5), E359–E386.
Hidalgo, M. (2010). Pancreatic cancer. New England Journal of Medicine, 362, 1605–1617. doi:10.1056/NEJMra0901557.
International Union against Cancer. (2010). TNM classification of malignant tumours (7th ed.). Chichester, West Sussex, UK; Hoboken, NJ: Wiley-Blackwell.
Seufferlein, T., Bachet, J. B., Van Cutsem, E., et al. (2012). Pancreatic adenocarcinoma: ESMO–ESDO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology, 23(Suppl 7), vii33–vii40. doi:10.1093/annonc/mds224.
Diouf, M., Filleron, T., Barbare, J.-C., et al. (2013). The added value of quality of life (QoL) for prognosis of overall survival in patients with palliative hepatocellular carcinoma. Journal of Hepatology, 58, 509–521. doi:10.1016/j.jhep.2012.11.019.
Bernhard, J., Dietrich, D., Glimelius, B., et al. (2010). Estimating prognosis and palliation based on tumour marker CA 19-9 and quality of life indicators in patients with advanced pancreatic cancer receiving chemotherapy. British Journal of Cancer, 103, 1318–1324. doi:10.1038/sj.bjc.6605929.
Gourgou-Bourgade, S., Bascoul-Mollevi, C., Desseigne, F., et al. (2013). Impact of FOLFIRINOX compared with gemcitabine on quality of life in patients with metastatic pancreatic cancer: Results from the PRODIGE 4/ACCORD 11 randomized trial. Journal of Clinical Oncology, 2013(31), 23–29. doi:10.1200/JCO.2012.44.4869.
Braun, D. P., Gupta, D., & Staren, E. D. (2013). Longitudinal health-related quality of life assessment implications for prognosis in stage IV pancreatic cancer. Pancreas, 42, 254–259. doi:10.1097/MPA.0b013e31825b9f56.
Steyerberg, E. W. (2008). Clinical prediction models: A practical approach to development, validation, and updating (1st ed.). New York, NY: Springer-Verlag New York Inc.
Trouilloud, I., Dupont-Gossard, A.-C., Malka, D., et al. (2014). Fixed-dose rate gemcitabine alone or alternating with FOLFIRI.3 (irinotecan, leucovorin and fluorouracil) in the first-line treatment of patients with metastatic pancreatic adenocarcinoma: An AGEO randomised phase II study (FIRGEM). European Journal of Cancer, 50, 3116–3124. doi:10.1016/j.ejca.2014.09.015.
Ishwaran, H., Kogalur, U. B., Blackstone, E. H., et al. (2008). Random survival forests. The Annals of Applied Statistics, 2, 841–860. doi:10.1214/08-AOAS169.
Bonnetain, F., Bonsing, B., Conroy, T., et al. (2014). Guidelines for time-to-event end-point definitions in trials for pancreatic cancer. Results of the DATECAN initiative (Definition for the Assessment of Time-to-event End-points in CANcer trials). European Journal of Cancer, 50, 2983–2993. doi:10.1016/j.ejca.2014.07.011.
Chen, X., & Ishwaran, H. (2014). Random forests for genomic data analysis. Genomics, 99, 323–329. doi:10.1016/j.ygeno.2012.04.003.
Van Steen, K., Curran, D., Kramer, J., et al. (2002). Multicollinearity in prognostic factor analyses using the EORTC QLQ-C30: Identification and impact on model selection. Statistics in Medicine, 21, 3865–3884. doi:10.1002/sim.1358.
Strobl, C., Boulesteix, A.-L., Kneib, T., et al. (2008). Conditional variable importance for random forests. BMC Bioinformatics, 9, 307. doi:10.1186/1471-2105-9-307.
Ishwaran, H., Kogalur, U. B., Gorodeski, E. Z., et al. (2010). High-dimensional variable selection for survival data. Journal of American Statistical Association, 105, 205–217. doi:10.1198/jasa.2009.tm08622.
Pang, H., & Jung, S.-H. (2013). Sample size considerations of prediction-validation methods in high-dimensional data for survival outcomes. Genetic Epidemiology, 37, 276–282. doi:10.1002/gepi.21721.
Hothorn, T., Hornik, K., & Zeileis, A. (2006). Unbiased recursive partitioning: A conditional inference framework. Journal of Computational and Graphical Statistics, 15, 651–674. doi:10.1198/106186006X133933.
Harrell, F. E., Lee, K. L., & Mark, D. B. (1996). Multivariable prognostic models: Issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in Medicine, 15, 361–387. doi:10.1002/(SICI)1097-0258(19960229)15:4<361:AID-SIM168>3.0.CO;2-4.
Schemper, M. (2003). Predictive accuracy and explained variation. Statistics in Medicine, 22, 2299–2308. doi:10.1002/sim.1486.
Diouf, M., Bonnetain, F., Barbare, J.-C., et al. (2015). Optimal Cut Points for Quality of Life Questionnaire-Core 30 (QLQ-C30) Scales: Utility for clinical trials and updates of prognostic systems in advanced hepatocellular carcinoma. The Oncologist, 20(1), 62–71.
Braun, D. P., Gupta, D., Grutsch, J. F., et al. (2011). Can changes in health related quality of life scores predict survival in stages III and IV colorectal cancer? Health and Quality of Life Outcomes, 9, 62. doi:10.1186/1477-7525-9-62.
Haas, M., Heinemann, V., Kullmann, F., et al. (2013). Prognostic value of CA 19-9, CEA, CRP, LDH and bilirubin levels in locally advanced and metastatic pancreatic cancer: Results from a multicenter, pooled analysis of patients receiving palliative chemotherapy. Journal of Cancer Research and Clinical Oncology, 139, 681–689. doi:10.1007/s00432-012-1371-3.
Robinson, D. W., Eisenberg, D. F., Cella, D., et al. (2008). The prognostic significance of patient-reported outcomes in pancreatic cancer cachexia. The Journal of Supportive Oncology, 6, 283–290.
Gotay, C. C., Kawamoto, C. T., Bottomley, A., et al. (2008). The prognostic significance of patient-reported outcomes in cancer clinical trials. Journal of Clinical Oncology, 26, 1355–1363. doi:10.1200/JCO.2007.13.3439.
Mauer, M., Bottomley, A., Coens, C., et al. (2008). Prognostic factor analysis of health-related quality of life data in cancer: A statistical methodological evaluation. Expert Review of Pharmacoeconomics & Outcomes Research, 8, 179–196. doi:10.1586/14737167.8.2.179.
Funding
This study did not receive any direct funding. However, we used data from the FIRGEM phase II trial which was funded by Pfizer and two French associations: AGEO (Association des Gastro-Entérologues Oncologues) and AROLD (Association pour la Recherche en Oncologie Digestive).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declared no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Diouf, M., Filleron, T., Pointet, AL. et al. Prognostic value of health-related quality of life in patients with metastatic pancreatic adenocarcinoma: a random forest methodology. Qual Life Res 25, 1713–1723 (2016). https://doi.org/10.1007/s11136-015-1198-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-015-1198-x