Abstract
Purpose
The Quality-of-life (QOL) Disease Impact Scale (QDIS®) standardizes the content and scoring of QOL impact attributed to different diseases using item response theory (IRT). This study examined the IRT invariance of the QDIS-standardized IRT parameters in an independent sample.
Method
The differential functioning of items and test (DFIT) of a static short-form (QDIS-7) was examined across two independent sources: patients hospitalized for acute coronary syndrome (ACS) in the TRACE-CORE study (N = 1,544) and chronically ill US adults in the QDIS standardization sample. “ACS-specific” IRT item parameters were calibrated and linearly transformed to compare to “standardized” IRT item parameters. Differences in IRT model-expected item, scale and theta scores were examined. The DFIT results were also compared in a standard logistic regression differential item functioning analysis.
Results
Item parameters estimated in the ACS sample showed lower discrimination parameters than the standardized discrimination parameters, but only small differences were found for thresholds parameters. In DFIT, results on the non-compensatory differential item functioning index (range 0.005–0.074) were all below the threshold of 0.096. Item differences were further canceled out at the scale level. IRT-based theta scores for ACS patients using standardized and ACS-specific item parameters were highly correlated (r = 0.995, root-mean-square difference = 0.09). Using standardized item parameters, ACS patients scored one-half standard deviation higher (indicating greater QOL impact) compared to chronically ill adults in the standardization sample.
Conclusion
The study showed sufficient IRT invariance to warrant the use of standardized IRT scoring of QDIS-7 for studies comparing the QOL impact attributed to acute coronary disease and other chronic conditions.




Similar content being viewed by others
Abbreviations
- ACS:
-
Acute coronary syndrome
- ACS-LT:
-
ACS-specific linearly transformed
- CAT:
-
Computerized adaptive testing
- CDIF:
-
Compensatory differential item functioning
- CFA:
-
Confirmatory factor analysis
- DFIT:
-
Differential functioning of items and tests
- DICAT:
-
The computerized adaptive Assessment of disease impact project
- DIF:
-
Differential item functioning
- DTF:
-
Differential test (scale) functioning
- GPCM:
-
Generalized partial credit model
- ICC:
-
Item characteristic curve
- IPD:
-
Item parameter drift
- IRT:
-
Item response theory
- MLHFQ:
-
Minnesota Living with Heart Failure Questionnaire
- NCDIF:
-
Non-compensatory differential item functioning
- PRO:
-
Patient-reported outcome
- PROMIS:
-
Patient Reported Outcomes Measurement Information System
- QDIS® :
-
Quality-of-life Disease Impact Scale
- QDIS-7:
-
7-item short-form of QDIS®
- QOL:
-
Quality-of-life
- RMSD:
-
Root-mean-square difference
- SAQ:
-
Seattle Angina Questionnaire
- TCC:
-
Test characteristic curve
- TRACE-CORE:
-
The Transitions, Risks, and Actions in Coronary Events-Center for Outcomes Research and Education project
References
Ware, J. E, Jr, Kemp, J. P., Buchner, D. A., et al. (1998). The responsiveness of disease-specific and generic health measures to changes in the severity of asthma among adults. Quality of Life Research, 7(3), 235–244.
De Boer, A. G., Spruijt, R. J., Sprangers, M. A., & de Haes, J. C. (1998). Disease-specific quality of life: is it one construct? Quality of Life Research, 7(2), 135–142.
Spertus, J. A., Winder, J. A., Drewhurst, T. A., et al. (1995). Development and evaluation of the Seattle Angina Questionnaire: a new functional status measure for coronary artery disease. Journal of the American College of Cardiology, 25, 333–341.
Rector, T. S., Kubo, S. H., & Cohn, J. N. (1987). Patients’ self-assessment of their congestive heart failure. Part 2: content, reliability and validity of a new measure, the Minnesota Living with Heart Failure questionnaire. Heart Failure, 3, 198–209.
Stewart, A. L., Greenfield, S., Hays, R. D., et al. (1989). Functional status and well-being of patients with chronic conditions. Results from the Medical Outcomes Study. JAMA, 262, 907–913.
Ware, J. E, Jr, Harrington, M., Guyer, R., & Boulanger, R. (2012). A system for integrating generic and disease-specific patient-reported outcome (PRO) measures. Patient Reported Outcomes Newsletter, 48(Fall), 2–4.
Ware, J. E, Jr, Gandek, B., & Guyer, R. (2014). Measuring disease-specific quality of life (QOL) impact: A manual for users of the QOL Disease Impact Scale (QDIS ® ). Worcester, MA: JWRG Incorporated.
Ware, J. E. Jr., Guyer, R., Gandek, B., Deng, N. Standardizing disease-specific quality of life (QOL) impact measures: Development and initial evaluation of the QOL Disease Impact Scale (QDIS ® ) (submitted).
Hambleton, R. K., Swaminathan, H., & Rogers, H. J. (1991). Fundamentals of item response theory. Newbury Park, CA: Sage.
Swaminathan, H., & Rogers, H. J. (1990). Detecting differential item functioning using logistic regression procedures. Journal of Educational Measurement, 27, 361–370.
Raju, N. S., van der Linden, W. J., & Fleer, P. F. (1995). IRT-based internal measures of differential functioning of items and tests. Applied Psychological Measurement, 19, 353–368.
Oldroyd, J. C., Cyril, S., Wijayatilaka, B. S., et al. (2013). Evaluating the impact of depression, anxiety & autonomic function on health related quality of life, vocational functioning and health care utilisation in acute coronary syndrome patients: the ADVENT study protocol. BMC Cardiovascular Disorders, 13, 103.
Kim, M. J., Jeon, D. S., Gwon, H. C., et al. (2013). Health-related quality-of-life after percutaneous coronary intervention in patients with UA/NSTEMI and STEMI: The Korean multicenter registry. Journal of Korean Medical Science, 28(6), 848–854.
Waring, M. E., McManus, R. H., Saczynski, J. S., et al. (2012). Transitions, risks, and actions in coronary events-center for outcomes research and education (TRACE-CORE): Design and rationale. Circulation Cardiovascular Quality and Outcomes, 5(5), e44–e50.
Howard, K. I., & Forehand, G. G. (1962). A method for correcting item-total correlations for the effect of relevant item inclusion. Educational and Psychological Measurement, 22, 731–735.
Cronbach, L. J. (1951). Coefficient alpha and the internal structure of tests. Psychometrika, 16(3), 297–334.
Oshima, T. C., & Morris, S. B. (2008). Raju’s differential functioning of items and tests (DFIT). Educational Measurement: Issues and Practice, 27, 43–50.
Flowers, C. P., Oshima, T. C., & Raju, N. S. (1999). A description and demonstration of the polytomous-DFIT framework. Applied Psychological Measurement, 23, 309–326.
Bock, R. D. (1972). Estimating item parameters and latent ability when responses are scored in two or more nominal categories. Psychometrika, 37, 29–51.
Swaminathan, H., Hambleton, R. K., & Rogers, H. J. (2007). Assessing the fit of item response theory models. In C. R. Rao & S. Sinharay (Eds.), Handbook of Statistics: Psychometrics (pp. 683–718). London: Elsevier Publishing Co.
Hambleton, R. K., & Han, N. (2005). Assessing the fit of IRT models to educational and psychological test data: A five step plan and several graphical displays. In W. R. Lenderking & D. Revicki (Eds.), Advances in health outcomes research methods, measurement, statistical analysis, and clinical applications (pp. 57–78). Washington: Degnon Associates.
Muraki, E., & Bock, R. D. (2003). PARSCALE 4: IRT item analysis and test scoring for rating scale data [computer program]. Chicago, IL: Scientific Software.
Liang, T., Han, K. T., & Hambleton, R. K. (2009). ResidPlots-2: Computer software for IRT graphical residual analyses. Applied Psychological Measurement, 33(5), 411–412.
De Ayala, R. J. (2009). The theory and practice of item response theory. New York: The Guilford Press.
Stocking, M. L., & Lord, F. M. (1983). Developing a common metric in item response theory. Applied Psychological Measurement, 7, 201–210.
Kolen, M. J., & Brennan, R. L. (2004). Test equating, scaling and linking: Methods and practices (2nd ed.). New York: Springer.
Sukin, Tia M. (2010). Item parameter drift as an indication of differential opportunity to learn: An exploration of item flagging methods & accurate classification of examinees. Doctoral dissertation. http://scholarworks.umass.edu/open_access_dissertations/301 Accessed 13 March 2014.
Raju, N. S. (1988). The area between two item characteristic curves. Psychometrika, 53, 495–502.
Fleer, P. F. (1993). A Monte Carlo assessment of a new measure of item and test bias. Doctoral dissertation, Illinois Institute of Technology. Dissertation Abstracts International, 54, 2266.
Raju, N. (2000). Notes accompanying the differential functioning of items and tests (DFIT) computer program. Chicago: Illinois Institute of Technology.
Teresi, J. A., Ocepek-Welikson, K., Kleinman, M., et al. (2007). Evaluating measurement equivalence using the item response theory log-likelihood ratio (IRTLR) method to assess differential item functioning (DIF): Applications (with illustrations) to measures of physical functioning ability and general distress. Quality of Life Research, 16, 43–68.
Raju, N. S. (1990). Determining the significance of estimated signed and unsigned areas between two item response functions. Applied Psychological Measurement, 14, 197–207.
Zumbo, B. D. (1999). A handbook on the theory and methods of differential item functioning (DIF): Logistic regression modeling as a unitary framework for binary and Likert-type (Ordinal) item scores. Ottawa ON: Directorate of Human Resources Research and Evaluation, Department of National Defense.
Crane, P. K., van Belle, G., & Larson, E. B. (2004). Test bias in a cognitive test: differential item functioning in the CASI. Statistics in Medicine, 23(2), 241–256.
Reeve, B., Hays, R. D., Bjorner, J., et al., on behalf of the PROMIS cooperative group. (2007). Psychometric evaluation and calibration of health–related quality of life item banks: Plans for the Patient-Reported Outcome Measurement Information System (PROMIS). Medical Care, 45(5), S22–S31.
Rose, M., Bjorner, J. B., Becker, J., Fries, J. F., & Ware, J. E. (2008). Evaluation of a preliminary physical function item bank supports the expected advantages of the Patient-Reported Outcomes Measurement Information System (PROMIS). Journal of Clinical Epidemiology, 61, 17–33.
Reise, S. P., Widaman, K. F., & Pugh, R. H. (1993). Confirmatory factor analysis and item response theory: Two approaches for exploring measurement invariance. Psychological Bulletin, 114(3), 552–566.
Raju, N. S., Laffitte, L. J., & Byrne, B. M. (2002). Measurement equivalence: a comparison of methods based on confirmatory factor analysis and item response theory. Journal of Applied Psychology, 87(3), 517–529.
Gregorich, S. E. (2006). Do self-report instruments allow meaningful comparisons across diverse population groups? Testing measurement invariance using the confirmatory factor analysis framework. Medical Care, 44(11 Suppl 3), S78–S94.
Wong, C. K., Lam, C. L., & Mulhern, B. (2013). Measurement invariance of the Functional Assessment of Cancer Therapy—Colorectal quality-of-life instrument among modes of administration. Quality of Life Research, 22, 1415–1426.
Bjørner, J. B., Rose, M., Gandek, B., et al. (2014). Method of administration of PROMIS scales did not significantly impact score level, reliability, or validity. Journal of Clinical Epidemiology, 67(1), 108–113.
Alonso, J., Ferrer, M., Gandek, B., et al. (2004). Health-related quality of life associated with chronic conditions in eight countries: results from the International Quality of Life Assessment (IQOLA) Project. Quality of Life Research, 13, 283–298.
Patrick, D. L., & Deyo, R. A. (1989). Generic and disease-specific measures in assessing health status and quality of life. Medical Care, 27(3 Suppl), S217–S232.
Ware, J. E, Jr, Guyer, R., Harrington, M., & Boulanger, R. (2012). Evaluation of a more comprehensive survey item bank for standardizing disease-specific impact comparisons across chronic conditions. Quality of Life Research, 21(1 Suppl), 27–28.
R Core Team (2014). R: A language and environment for statistical computing. R foundation for statistical computing, Vienna, Austria. http://www.R-project.org/.
Candell, G. L., & Drasgow, F. (1988). An iterative procedure for linking metrics and assessing item bias in item response theory. Applied Psychological Measurement, 12, 253–260.
Hidalgo-Montesinos, M. D., & Lopez-Pina, J. A. (2002). Two-stage equating in differential item functioning detection under the graded response model with the Raju area measures and the Lord statistic. Educational and Psychological Measurement, 62, 32–44.
Bolt, D. M. (2002). A Monte Carlo comparison of parametric and nonparametric polytomous DIF detection methods. Applied Measurement in Education, 2, 113–141.
Oshima, T. C., Raju, N. S., & Nanda, A. O. (2006). A new method for assessing the statistical significance in the differential functioning of items and tests (DFIT) framework. Journal of Educational Measurement, 43, 1–17.
Bjorner, J. B., Rose, M., Gandek, B., Stone, A. A., Junghaenel, D. U., & Ware, J. E, Jr. (2013). Difference in method of administration did not significantly impact item response: An IRT-based analysis from the Patient-Reported Outcomes Measurement Information System initiative. Quality of Life Research, 23, 217–227.
Choi, S. W., Gibbons, L. E., & Crane, P. K. (2011). Lordif: An R Package for detecting differential item functioning using iterative hybrid ordinal logistic regression/item response theory and monte carlo simulations. Journal of Statistical Software, 39(8), 1–30.
Acknowledgments
TRACE-CORE is supported by the National Institutes of Health National Heart, Lung, and Blood Institute (1U01HL105268). DICAT is supported by the National Institutes of Health National Institute of Aging (2R44AG025589). Partial salary support was provided by TRACE-CORE and PhRMA foundation Research Starter Grant (M.D.A.). Additional support was provided by NIH Grant KL2TR000160 (M.E.W.). The authors are very grateful for the editor and reviewers’ comments and personal communication with Jakob Bjørner.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: illustration of calculations
Item response theory indeterminacy
The logit function in an IRT model is defined by
where P i (θ j ) is the probability of endorsing item response category i for person j, a i and b i are the item discrimination and threshold parameters for response category i, respectively, and θ j is the IRT-based theta score for person j. The logit will preserve the same value if a i , θ j , and bi were replaced by \(a_{i}^{'}\), \(\theta_{j}^{'}\), and \(b_{i}^{'}\), respectively, which follow a set of linear transformations of
where A is the slope and B is the intercept of the linear transformations.
Slope (A) and intercept (B) of linear transformation using IRT theta scores
where σ (θ ST) and σ (θ ACS) are the SD of the IRT scores of ACS patients using the standardized (θ ST) and the ACS-specific (θ ACS) IRT item parameters, respectively. μ (θ ST) and μ (θ ACS) are the means of the IRT scores.
Non-compensatory differential item functioning (NCDIF)
where S ST,i,j is the IRT model-expected item response score of item i for respondent j using the standardized IRT item parameters. S ACS,i,j is the IRT model-expected item response score of item i for respondent j using the ACS-LT IRT item parameters. N is the sample size of ACS patients.
Test differential functioning (DTF)
where TSST,j is the IRT model-expected scale(test) score for respondent j using the standardized IRT item parameters. TSACS,j is the IRT model-expected scale(test) score for respondent j using the ACS-LT IRT item parameters. N is the sample size of ACS patients.
Compensatory differential item functioning (CDIF)
where d i is the difference in the IRT model-expected item response score of item i between the standardized and the ACS-LT IRT item parameters. D is the difference in IRT model-expected scale score between the standardized and the ACS-LT IRT item parameters. COV(d i , D) is the covariance of d i and D. μ (d i ) and μ (D) are their means, respectively. Of note that DTF is equivalent to the sum of CDIF i added across all the items: \({\text{DTF}} = \mathop \sum \nolimits_{i = 1}^{I} {\text{CDIF}}_{i}\).
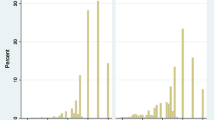
Appendix 2
Raw residual plots of fitting the IRT model in the ACS patients for the QDIS-7 (ordered by row from Item 1 to Item 7)

Rights and permissions
About this article
Cite this article
Deng, N., Anatchkova, M.D., Waring, M.E. et al. Testing item response theory invariance of the standardized Quality-of-life Disease Impact Scale (QDIS®) in acute coronary syndrome patients: differential functioning of items and test. Qual Life Res 24, 1809–1822 (2015). https://doi.org/10.1007/s11136-015-0916-8
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11136-015-0916-8