Abstract
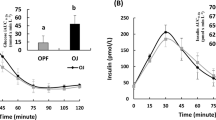
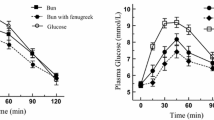
An aqueous filtered molasses concentrate (FMC) sourced from sugar cane was used as a functional ingredient in a range of carbohydrate-containing foods to reduce glycaemic response. When compared to untreated controls, postprandial glucose responses in the test products were reduced 5–20 %, assessed by accredited glycaemic index (GI) testing. The reduction in glucose response in the test foods was dose-dependent and directly proportional to the ratio of FMC added to the amount of available carbohydrate in the test products. The insulin response to the foods was also reduced with FMC addition as compared to untreated controls. Inclusion of FMC in test foods did not replace any formulation ingredients; it was incorporated as an additional ingredient to existing formulations.
Filtered molasses concentrate, made by a proprietary and patented process, contains many naturally occurring compounds. Some of the identified compounds are known to influence carbohydrate metabolism, and include phenolic compounds, minerals and organic acids. FMC, sourced from a by-product of sugar cane processing, shows potential as a natural functional ingredient capable of modifying carbohydrate metabolism and contributing to GI reduction of processed foods and beverages.


Similar content being viewed by others
Abbreviations
- CE:
-
Catechin equivalent (non-specific measure of phenolic activity)
- FMC:
-
Filtered molasses concentrate
- GI:
-
Glycemic index (measurement of 2 h postprandial glycemic response relative to pure glucose)
- II:
-
Insulinemic index or response (measurement of 2 hour postprandial insulin response relative to glucose)
- ORAC:
-
Oxygen-radical absorbance capacity (measure of antioxidant activity)
- pmol/L:
-
Picomole per litre
References
Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87:4–14
Larsen TM, Dalskov S-M, van Baak M, Jebb SA, Papadaki A, Pfeiffer AFH, Martinez A, Handijieva-Darlenska T, Kunešová M, Pihlsgård M, Stender S, Holst C, Saris WHM, Astrup A, for the Diogenes Project (2010) Diets with high or low protein content and glycaemic index for weight-loss maintenance. N Engl J Med 363:2102–2113
Brand-Miller J, Hayne S, Petocz P, Colagiuri S (2003) Low–glycaemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diabetes Care 26:2261–2267
Hsieh C-W, Cheng J-Y, Wang T-H, Wang H-J, Ho W-J (2014) Hypoglycaemic effects of Ajuga extract in vitro and in vivo. J Funct Foods 6:224–230
Thompson LU, Yoon JH, Jenkins DJA, Wolever TMS, Jenkins AL (1984) Relationship between polyphenol intake and blood glucose response of normal and diabetic individuals. Am J Clin Nutr 39:745–751
Hanhineva K, Törrönen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkänen H, Poutanen K (2010) Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci 11:1365–1402
Paolisso G, Barbagallo M (1997) Hypertension, diabetes mellitus, and insulin resistance: the role of intracellular magnesium. Am J Hypertens 10:346–355
Pikilidou MI, Lasaridis AN, Sarafidis PA, Befani CD, Koliakos GG, Tziolas IM, Kazakos KA, Yovos JG, Nilsson PM (2009) Insulin sensitivity increase after calcium supplementation and change in intraplatelet calcium and sodium-hydrogen exchange in hypertensive patients with type 2 diabetes. Diabet Med 26:211–219
Chatterjee R, Colangelo LA, Yeh HC, Anderson CA, Daviglus ML, Liu K, Brancati FL (2012) Potassium intake and risk of incident type 2 diabetes mellitus: the coronary artery risk development in young adults (CARDIA) study. Diabetologia 55:1295–1303
Heianza Y, Hara S, Arase Y, Saito K, Totsuka K, Tsuji H, Kodama S, Hsieh SD, Yamada N, Kosaka K, Sone H (2011) Low serum potassium levels and risk of type 2 diabetes: the Toranomon hospital health management center study 1 (TOPICS 1). Diabetologia 54:762–766
Liljeberg HGM, Lönner CH, Björck IME (1995) Sourdough fermentation or addition or organic acids or corresponding salts to bread improves nutritional properties of starch in healthy humans. J Nutr 125:1503–1511
von Post-Skagegård M, Vessby B, Karlström B (2006) Glucose and insulin responses in healthy women after intake of composite meals containing cod- milk- and soy protein. Eur J Clin Nutr 60:949–954
Collier GR, Greenberg GR, Wolever TMS, Jenkins DJA (1988) The acute effect of fat on insulin secretion. J Clin Endocrinol Metab 66:323–326
Min B, McClung AM, Chen M-H (2010) Phytochemicals and antioxidant capacities in rice brans of different color. J Food Sci 76:C117–C126
Chen L, Xin X, Zhang H, Yuan Q (2013) Phytochemical properties and antioxidant capacities of commercial raspberry varieties. J Funct Foods 5:508–515
Rothwell JA, Perez-Jimenez J, Neveu V, Medina-Remón A, M'Hiri N, García Lobato P, Manach C, Knox C, Eisner R, Wishart KS, Scalbert A (2013) Phenol-Explorer 3.0: a major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database, 2013, bat070
Munro JA, Mishra S, Venn B (2010) Baselines representing blood glucose clearance improve in vitro prediction of the glycaemic impact of customarily consumed food quantities. Br J Nutr 103:295–305
Macarulla MT, Martínez JA, Barcina Y, Larralde J (1989) Intestinal absorption of D-galactose in the presence of extracts from Phaseolus vulgaris hulls. Plant Foods Hum Nutr 39:359–367
Kobayashi Y, Suzuki M, Satsu H, Soichi A, Hara Y, Suzuki K, Miyamoto Y, Shimizu M (2000) Green tea polyphenols inhibit the sodium-dependent glucose transporter of intestinal epithelial cells by a competitive mechanism. J Agric Food Chem 48:5618–5623
Kwon O, Eck P, Chen S, Corpe CP, Lee J-H, Kruhlak M, Levine M (2007) Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J 21:366–377
Eid HM, Martineau LC, Saleem A, Muhammad A, Vallerand D, Benhaddou-Andaloussi A, Nistor L, Afshar A, Arnason JT, Haddad PS (2010) Stimulation of AMP-activated protein kinase and enhancement of basal glucose uptake in muscle cells by quercetin and quercetin glycosides, active principles of the antidiabetic medicinal plant Vaccinium vitis-idaea. Mol Nutr Food Res 54:991–1003
Park CE, Kim M-J, Lee JH, Min B-I, Bae H, Choe W, Kim SS, Ha J (2007) Resveratrol stimulates glucose transport in C2C12 myotubes by activating AMP-activated protein kinase. Exp Mol Med 39:222–229
Jung KH, Choi HS, Kim DH, Han MY, Chang UJ, Yim S-V, Song BC, Kim C-H, Kang SA (2008) Epigallocatechin gallate stimulates glucose uptake through the phosphatidylinositol 3-kinase-mediated pathway in L6 rat skeletal muscle cells. J Med Food 11:429–434
Cao H, Polansky MM, Anderson RA (2007) Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3 T3-L1 adipocytes. Arch Biochem Biophys 459:214–222
Montagut G, Onnockx S, Vaqué M, Bladé C, Blay M, Fernández-Larrea J, Pujadas G, Salvadó MJ, Arola L, Pirson I, Ardévol A, Pinent M (2010) Oligomers of grape-seed procyanidin extract activate the insulin receptor and key targets of the insulin signaling pathway differently from insulin. J Nutr Biochem 21:476–481
Kim D-O, Jeong SW, Lee CY (2003) Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem 81:321–326
Marinova D, Ribarova F, Atanassova M (2005) Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J Chem Technol Metall 40:255–260
Cao G, Alessio H, Cutler R (1993) Oxygen-radical absorbance capacity (ORAC) assay for antioxidants. Free Radic Biol Med 14:303–311
Li H, Song F, Xing J, Tsao R, Liu Z, Liu S (2009) Screening and structural characterization of α-glucosidase inhibitors from hawthorn leaf flavonoids extract by ultrafiltration LC-DAD-MSn and SORI-CID FTICR MS. J Am Soc Mass Spectrom 20:1496–1503
Nickavar B, Amin G (2010) Bioassay-guided separation of an alpha-amylase inhibitor anthocyanin from Vaccinium arctostaphylos berries. Z Naturforsch 65:567–570
Pari L, Srinivasan S (2010) Antihyperglycemic effect of diosmin on hepatic key enzymes of carbohydrate metabolism in streptozotocin-nicotinamide-induced diabetic rats. Biomed Pharmacother 64:477–481
Choo CY, Sulong NY, Man F, Wong TW (2012) Vitexin and isovitexin from the leaves of Ficus deltoidea with in-vivo α-glucosidase inhibition. J Ethnopharmacol 142:776–781
Acknowledgments
The authors would like to thank Fiona Atkinson of the Sydney University Glycaemic Index Research Service for extensive GI testing, analysis and reporting; Ria Setyabudi (formerly Horizon Science) for sample preparation and analysis, Gunter Kuhnle of the University of Reading for polyphenol determination, and Plant and Food Research New Zealand for in vitro digestive enzyme studies.
Conflict of Interest
The authors declare they have no conflict of interest.
Study subjects
The authors declare this research study involved human subjects. This study was conducted in accordance with the ethical principles that have their origins in the Declaration of Helsinki. The experimental procedures used in this study were in accordance with international standards for conducting ethical research with humans and were approved by the Human Research Ethics Committee of Sydney University (approval number 08-2009/12029, valid August 13, 2009 – August 31, 2012). This study was performed between March 2011 and March 2012.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wright, A.G., Ellis, T.P. & Ilag, L.L. Filtered Molasses Concentrate from Sugar Cane: Natural Functional Ingredient Effective in Lowering the Glycaemic Index and Insulin Response of High Carbohydrate Foods. Plant Foods Hum Nutr 69, 310–316 (2014). https://doi.org/10.1007/s11130-014-0446-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-014-0446-5