Abstract
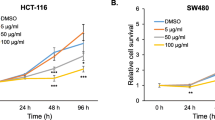
Melissa officinalis L. (Lamiaceae) is consumed as a traditional herbal tea in the Mediterranean region. The cytotoxic effect of the 50% ethanolic and aqueous extract, determined by the MTT and NR assays, was evaluated in vitro on Human Colon Cancer Cell Line (HCT-116), using Triton 10% as positive control. The 50% ethanolic extract showed significant differences after 72 h of treatment, reducing cell proliferation to values close to 40%, even the lowest dose tested (5 μg/ml). In the MTT assay, the same extract caused the lowest cell viability with 13% at a concentration of 1,000 μg/ml after 72 h of treatment, being a value lower than Triton 10%. The antioxidant activity was also confirmed evaluating the capacity of the extracts to scavenge ABTS and DPPH radicals, and IC50 values were highly correlated with the total phenolic and flavonoid content. Bioassay guided fractionation led to the isolation of an anti-proliferative compound, rosmarinic acid. Its structural elucidation was performed by HPLC/DAD/ESI/MS analysis. High dose of rosmarinic acid (1,000 μg/ml) was clearly cytotoxic against HCT-116 cells, with a significant decrease in cell number since the earliest time point (24 h).


Similar content being viewed by others
Abbreviations
- AQE:
-
Aqueous extract
- HAE:
-
50% ethanolic extract
- RA:
-
Rosmarinic acid
- TFC:
-
Total flavonoid content
- TPC:
-
Total phenolic content
References
Cancer. National Cancer Institute (2009) www.cancer.gov/cancertopics/commoncancers
Gordaliza M (2009) Natural products as leads to anticancer drugs. Clin Transl Oncol 9:767–776
Saklani A, Kutty SK (2008) Plant-derived compounds in clinical trials. Drug Discov Today 13:161–171
Wattenberg LW (1998) Chemoprevention of carcinogenesis by minor dietary constituents: Symposium introduction. Pharm Biol 36:6–7
Yang CS, Chung JY, Yang G, Chhabra SK, Lee MJ (2000) Tea and tea polyphenols in cancer prevention. J Nutr 130:472S–478S
Bisset NG (1994) Herbal drugs and phytopharmaceuticals: a handbook for practice scientific basis. CRC Press, Stuttgart, pp 329–332
de Sousa AC, Alviano DS, Blank AF, Alves PB, Alviano CS, Gattass CR (2004) Melissa officinalis L. essential oil: Antitumoral and antioxidant activities. J Pharm Parmacol 56:677–681
Kucera LS, Cohen RA, Herrmann EC (1965) Antiviral activities of extracts of the lemon balm plant. Ann NY Acad Sci 130:474–482
López V, Martín S, Gómez-Serranillos MP, Carretero ME, Jäger AK, Calvo MI (2009) Neuroprotective and neurological properties of Melissa officinalis. Neurochem Res 34:1955–1961
López V, Akerreta S, Casanova E, Garcia-Mina JM, Cavero RY, Calvo MI (2007) In vitro antioxidant and anti-rhizopus activities of Lamiaceae herbal extracts. Plant Foods Hum Nutr 62:151–155
García-Iñiguez de Cirano M, Larequi E, Rehecho S, Calvo MI, Cavero RY, Navarro-Blasco I, Astiasarán I, Ansorena D (2010) Selenium, iodine, ω-3 PUFA and natural antioxidant from Melissa officinalis L.: A combination of components from healthier dry fermented sausages formulation. Meat Sci 85:274–279
García-Iñiguez de Ciriano M, Rehecho S, Calvo MI, Cavero RY, Navarro I, Astiasarán I, Ansorena D (2010) Effect of lyophilized water extracts of Melissa officinalis on the stability of algae and linseed oil-in-water emulsion to be used as a functional ingredient in meat products. Meat Sci 85:373–377
López V, Akerreta S, Casanova E, García-Mina JM, Cavero RY, Calvo MI (2008) Screening of Spanish medicinal plants for antioxidant activities. Pharm Biol 46:602–609
López V, Jäger AK, Akerreta S, Cavero RY, Calvo MI (2010) Antioxidant activity and phenylpropanoids of Phlomis lychnitis L.: A traditional herbal tea. Plant Foods Hum Nutr 65:179–185
Pueyo IU, Calvo MI (2009) Assay conditions and validation of a new UV spectrophotometric method using microplates for the determination of polyphenol content. Fitoterapia 80:465–467
Rehecho S, Hidalgo O, García-Iñiguez de Cirano M, Navarro I, Astiasarán I, Ansorena D, Cavero RY, Calvo MI (2011) Chemical composition, mineral content and antioxidant activity of Verbena officinalis L. LWT-Food Sci Technol 44:875–882
Hohmann J, Zupko I, Redei D, Csanyi M, Falkay G, Mathe I, Janicsak G (1999) Protective effects of the aerial parts of Salvia officinalis, Melissa officinalis and Lavandula angustifolia and their constituents against enzyme-dependent and enzyme-independent lipid peroxidation. Planta Med 65:576–578
Ivanova D, Genova D, Chervenkov T, Yankova T (2005) Polyphenols and antioxidant capacity of Bulgarian medicinal plants. J Ethnopharmacol 96:145–150
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Borenfreunds E, Babich H, Martin-Alguacil N (1998) Comparisons of two in vitro cytotoxicity assays- The neutral red (NR) and tetrazolium MTT tests. Toxicol in Vitro 2:1–6
Ma PL, Zhao PR, Tian AQ, Zhang S (2006) The effect of Prunella vulgaris L. on Eca109 cells. J Basic Clin Oncol 19:199–200
Yesil-Celiktas O, Sevimli C, Bedir E, Vardar-Sukan F (2010) Inhibitory effects of rosemary extracts, carnosic acid and rosmarinic acid on the growth of various human cancer cell lines. Plant Foods Hum Nutr 65:158–163
Wang H, Provan GJ, Helliwel K (2004) Determination of rosmarinic acid and caffeic acid in aromatic herbs by HPLC. Food Chem 87:307–311
Petersen M, Simmonds MS (2003) Molecules of Interest. Rosmarinic acid. Phytochemistry 62:121–125
Osakabe N, Takano H, Sanbongi C, Yasuda A, Yanagisawa R, Inoue K, Yoshikawa T (2004) Anti-inflammatory and anti-allergic effect of rosmarinic acid (RA); inhibition of seasonal allergic rhinoconjunctivitis (SAR) and its mechanism. Biofactors 21:127–131
Swarup V, Ghosh J, Ghosh S, Saxena A, Basu A (2007) Antiviral and anti-inflammatory effects of rosmarinic acid in an experimental murine model of Japanese encephalitis. Antimicrob Agents Chemother 51:3367–3370
Xavier C, Lima C, Fernandes-Ferreira M, Pereira-Wilson C (2009) Salvia fruticosa, Salvia officinalis, and rosmarinic acid induced apoptosis and inhibit proliferation of human colorectal cell lines: The role in mapk/erk pathway. Nutr Cancer 61:564–571
Xu YC, Xu GL, Liu L, Xu DS, Liu JW (2010) Anti-invasion effect of rosmarinic acid via the extracellular signal-regulated kinase and oxidation-reduction pathway in Ls174-T cells. J Cell Biochem 111:370–379
Acknowledgements
We thank the “Proyecto AGL2008-01099/ALI” and “CONSOLIDER 2010-CARNISENUSA CSD2007-00016” (Ministerio de Ciencia e Innovación), and the “Plan Investigador de la Universidad de Navarra” (PIUNA) for their contribution to the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Encalada, M.A., Hoyos, K.M., Rehecho, S. et al. Anti-proliferative Effect of Melissa officinalis on Human Colon Cancer Cell Line. Plant Foods Hum Nutr 66, 328–334 (2011). https://doi.org/10.1007/s11130-011-0256-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11130-011-0256-y