Abstract
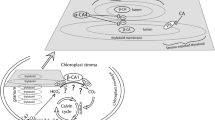
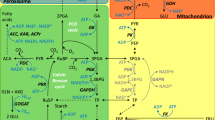
Chlamydomonas reinhardtii evolved a CO2-concentrating mechanism (CCM) because of the limited CO2 in its natural environment. One critical component of the C. reinhardtii CCM is the limiting CO2 inducible B (LCIB) protein. LCIB is required for acclimation to air levels of CO2. C. reinhardtii cells with a mutated LCIB protein have an ‘air-dier’ phenotype when grown in low CO2 conditions, meaning they die in air levels of CO2 but can grow in high and very low CO2 conditions. The LCIB protein functions together with its close homolog in C. reinhardtii, limiting CO2 inducible C protein (LCIC), in a hexameric LCIB-LCIC complex. LCIB has been proposed to act as a vectoral carbonic anhydrase (CA) that helps to recapture CO2 that would otherwise leak out of the chloroplast. Although both LCIB and LCIC are structurally similar to βCAs, their CA activity has not been demonstrated to date. We provide evidence that LCIB is an active CA using a Saccharomyces cerevisiae CA knockout mutant (∆NCE103) and an Arabidopsis thaliana βCA5 knockout mutant (βca5). We show that different truncated versions of the LCIB protein complement ∆NCE103, while the full length LCIB protein complements βca5 plants, so that both the yeast and plant mutants can grow in low CO2 conditions.







Similar content being viewed by others
Data Availability
All data supporting the findings of this study are available within the paper and within its supplementary materials published online. Any data not shown are available from the corresponding author, James V. Moroney, upon request.
References
Aguilera J, Van Dijken JP, De Winde JH, Pronk JT (2005) Carbonic anhydrase (Nce103p): an essential biosynthetic enzyme for growth of Saccharomyces cerevisiae at atmospheric carbon dioxide pressure. Biochem J 391(Pt 2):311–316. https://doi.org/10.1042/bj20050556
Brueggeman AJ, Gangadharaiah DS, Cserhati MF, Casero D, Weeks DP, Ladunga I (2012) Activation of the carbon concentrating mechanism by CO2 deprivation coincides with massive transcriptional restructuring in Chlamydomonas reinhardtii. Plant Cell 24(5):1860–1875. https://doi.org/10.1105/tpc.111.093435
DiMario RJ, Clayton H, Mukherjee A, Ludwig M, Moroney JV (2017) Plant carbonic anhydrases: structures, locations, evolution, and physiological roles. Mol Plant 10(1):30–46. https://doi.org/10.1016/j.molp.2016.09.001
Duanmu D, Wang Y, Spalding MH (2009) Thylakoid lumen carbonic anhydrase (CAH3) mutation suppresses air-dier phenotype of LCIB mutant in Chlamydomonas reinhardtii. Plant Physiol 149(2):929–937. https://doi.org/10.1104/pp.108.132456
Engel BD, Schaffer M, Kuhn Cuellar L, Villa E, Plitzko JM, Baumeister W (2015) Native architecture of the Chlamydomonas chloroplast revealed by in situ cryo-electron tomography. Elife. https://doi.org/10.7554/eLife.04889
Fukuzawa H, Miura K, Ishizaki K, Kucho KI, Saito T, Kohinata T, Ohyama K (2001) Ccm1, a regulatory gene controlling the induction of a carbon-concentrating mechanism in Chlamydomonas reinhardtii by sensing CO2 availability. Proc Natl Acad Sci U S A 98(9):5347–5352. https://doi.org/10.1073/pnas.081593498
Funke RP, Kovar JL, Weeks DP (1997) Intracellular carbonic anhydrase is essential to photosynthesis in Chlamydomonas reinhardtii at atmospheric levels of CO2 (demonstration via genomic complementation of the high-CO2-requiring mutant ca-1). Plant Physiol 114(1):237–244. https://doi.org/10.1104/pp.114.1.237
Gietz RD, Schiestl RH (2007) High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc 2(1):31–34. https://doi.org/10.1038/nprot.2007.13
Hines KM, Chaudhari V, Edgeworth KN, Owens TG, Hanson MR (2021) Absence of carbonic anhydrase in chloroplasts affects C(3) plant development but not photosynthesis. Proc Natl Acad Sci USA 118(33):e2107425118
Jin S, Sun J, Wunder T, Tang D, Cousins AB, Sze SK, Mueller-Cajar O, Gao Y-G (2016) Structural insights into the LCIB protein family reveals a new group of β-carbonic anhydrases. Proc Natl Acad Sci USA 113:14716–14721. https://doi.org/10.1073/pnas.1616294113
Karlsson J, Clarke AK, Chen ZY, Hugghins SY, Park YI, Husic HD, Moroney JV, Samuelsson G (1998) A novel alpha-type carbonic anhydrase associated with the thylakoid membrane in Chlamydomonas reinhardtii is required for growth at ambient CO2. Embo J 17(5):1208–1216. https://doi.org/10.1093/emboj/17.5.1208
Li X, Zhang R, Patena W, Gang SS, Blum SR, Ivanova N, Yue R, Robertson JM, Lefebvre PA, Fitz-Gibbon ST, Grossman AR, Jonikas MC (2016) An indexed, mapped mutant library enables reverse genetics studies of biological processes in Chlamydomonas reinhardtii. Plant Cell 28(2):367–387. https://doi.org/10.1105/tpc.15.00465
Li F-W, Nishiyama T, Waller M, Frangedakis E, Keller J, Li Z, Fernandez-Pozo N, Barker MS, Bennett T, Blázquez MA, Cheng S, Cuming AC, de Vries J, de Vries S, Delaux P-M, Diop IS, Harrison CJ, Hauser D, Hernández-García J, Kirbis A, Meeks JC, Monte I, Mutte SK, Neubauer A, Quandt D, Robison T, Shimamura M, Rensing SA, Villarreal JC, Weijers D, Wicke S, Wong GKS, Sakakibara K, Szövényi P (2020) Anthoceros genomes illuminate the origin of land plants and the unique biology of hornworts. Nat Plants 6(3):259–272. https://doi.org/10.1038/s41477-020-0618-2
Ma Y, Pollock SV, Xiao Y, Cunnusamy K, Moroney JV (2011) Identification of a novel gene, CIA6, required for normal pyrenoid formation in Chlamydomonas reinhardtii. Plant Physiol 156(2):884–896. https://doi.org/10.1104/pp.111.173922
Mackinder LCM, Chen C, Leib RD, Patena W, Blum SR, Rodman M, Ramundo S, Adams CM, Jonikas MC (2017) A spatial interactome reveals the protein organization of the algal CO2-concentrating mechanism. Cell 171(1):133-147.e114. https://doi.org/10.1016/j.cell.2017.08.044
Medina-Puche L, Castelló MJ, Canet JV, Lamilla J, Colombo ML, Tornero P (2017) β-carbonic anhydrases play a role in salicylic acid perception in Arabidopsis. PLoS ONE 12(7):e0181820. https://doi.org/10.1371/journal.pone.0181820
Miura K, Yamano T, Yoshioka S, Kohinata T, Inoue Y, Taniguchi F, Asamizu E, Nakamura Y, Tabata S, Yamato KT, Ohyama K, Fukuzawa H (2004) Expression profiling-based identification of CO2-responsive genes regulated by CCM1 controlling a carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Physiol 135(3):1595–1607. https://doi.org/10.1104/pp.104.041400
Moroney JV, Somanchi A (1999) How do algae concentrate CO2 to increase the efficiency of photosynthetic carbon fixation? Plant Physiol 119(1):9–16. https://doi.org/10.1104/pp.119.1.9
Moroney JV, Ynalvez RA (2007) Proposed carbon dioxide concentrating mechanism in Chlamydomonas reinhardtii. Eukaryot Cell 6(8):1251–1259. https://doi.org/10.1128/ec.00064-07
Moroney JV, Husic HD, Tolbert NE, Kitayama M, Manuel LJ, Togasaki RK (1989) Isolation and characterization of a mutant of Chlamydomonas reinhardtii deficient in the CO2 concentrating mechanism. Plant Physiol 89(3):897–903. https://doi.org/10.1104/pp.89.3.897
Moroney JV, Ma Y, Frey WD, Fusilier KA, Pham TT, Simms TA, DiMario RJ, Yang J, Mukherjee B (2011) The carbonic anhydrase isoforms of Chlamydomonas reinhardtii: intracellular location, expression, and physiological roles. Photosynth Res 109:133–149. https://doi.org/10.1007/s11120-011-9635-3
Mukherjee A, Lau CS, Walker CE, Rai AK, Prejean CI, Yates G, Emrich-Mills T, Lemoine SG, Vinyard DJ, Mackinder LCM, Moroney JV (2019) Thylakoid localized bestrophin-like proteins are essential for the CO2 concentrating mechanism of Chlamydomonas reinhardtii. Proc Natl Acad Sci U S A 116(34):16915–16920. https://doi.org/10.1073/pnas.1909706116
Rai AK, DiMario RJ, Kasili RW, Groszmann M, Cousins AB, Donze D, Moroney JV (2022) A rapid method for detecting normal or modified plant and algal carbonic anhydrase activity using Saccharomyces cerevisiae. Plants (basel). https://doi.org/10.3390/plants11141882
Spalding MH (2008) Microalgal carbon-dioxide-concentrating mechanisms: Chlamydomonas inorganic carbon transporters. J Exp Bot 59(7):1463–1473
Spalding MH, Spreitzer RJ, Ogren WL (1983) Reduced inorganic carbon transport in a CO2-requiring mutant of Chlamydomonas reinhardii. Plant Physiol 73(2):273–276. https://doi.org/10.1104/pp.73.2.273
Spalding MH, Van K, Wang Y, Nakamura Y (2002) Acclimation of Chlamydomonas to changing carbon availability. Funct Plant Biol 29(3):221–230. https://doi.org/10.1071/PP01182
Toyokawa C, Yamano T, Fukuzawa H (2020) Pyrenoid starch sheath is required for LCIB localization and the CO2-concentrating mechanism in green algae. Plant Physiol 182(4):1883–1893. https://doi.org/10.1104/pp.19.01587
Van K, Wang Y, Nakamura Y, Spalding MH (2001) Insertional mutants of Chlamydomonas reinhardtii that require elevated CO2 for survival. Plant Physiol 127(2):607–614. https://doi.org/10.1104/pp.010333
Wang Y, Spalding MH (2006) An inorganic carbon transport system responsible for acclimation specific to air levels of CO2 in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 103(26):10110–10115. https://doi.org/10.1073/pnas.0603402103
Wang Y, Spalding MH (2014a) Acclimation to very low CO2: Contribution of limiting CO2 inducible proteins, LCIB and LCIA, to inorganic carbon uptake in Chlamydomonas reinhardtii. Plant Physiol 166(4):2040–2050. https://doi.org/10.1104/pp.114.248294
Wang Y, Spalding MH (2014b) LCIB in the Chlamydomonas CO2-concentrating mechanism. Photosynth Res 121(2–3):185–192. https://doi.org/10.1007/s11120-013-9956-5
Wang Y, Stessman DJ, Spalding MH (2015) The CO2 concentrating mechanism and photosynthetic carbon assimilation in limiting CO2: how Chlamydomonas works against the gradient. Plant J 82(3):429–448
Weerasooriya HN, DiMario RJ, Rosati VC, Rai AK, LaPlace LM, Filoon VD, Longstreth DJ, Moroney JV (2022) Arabidopsis plastid carbonic anhydrase βCA5 is important for normal plant growth. Plant Physiol 190(4): 2173-2186. https://doi.org/10.1093/plphys/kiac451
Weigel D, Glazebrook J (2006) Transformation of Agrobacterium using the freeze-thaw method. CSH Protoc. https://doi.org/10.1101/pdb.prot4666
Xiang Y, Zhang J, Weeks DP (2001) The Cia5 gene controls formation of the carbon concentrating mechanism in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 98(9):5341–5346. https://doi.org/10.1073/pnas.101534498
Yamano T, Tsujikawa T, Hatano K, Ozawa S-i, Takahashi Y, Fukuzawa H (2010) Light and low-CO2-dependent LCIB–LCIC complex localization in the chloroplast supports the carbon-concentrating mechanism in Chlamydomonas reinhardtii. Plant Cell Physiol 51(9):1453–1468. https://doi.org/10.1093/pcp/pcq105
Yamano T, Toyokawa C, Shimamura D, Matsuoka T, Fukuzawa H (2022) CO2-dependent migration and relocation of LCIB, a pyrenoid-peripheral protein in Chlamydomonas reinhardtii. Plant Physiol 188(2):1081–1094. https://doi.org/10.1093/plphys/kiab528
Zhang X, Henriques R, Lin S-S, Niu Q-W, Chua N-H (2006) Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc 1(2):641–646. https://doi.org/10.1038/nprot.2006.97
Zhang T, Lei J, Yang H, Xu K, Wang R, Zhang Z (2011) An improved method for whole protein extraction from yeast Saccharomyces cerevisiae. Yeast 28(11):795–798. https://doi.org/10.1002/yea.1905
Zhu F, Saluja M, Dharni JS, Paul P et al (2021) PhenoImage: an open-source graphical user interface for plant image analysis. Plant Phenome J. https://doi.org/10.1002/ppj2.20015
Acknowledgements
This work was supported by the Realizing Improved Photosynthetic Efficiency (RIPE) initiative awarded to JVM by the University of Illinois, USA. RIPE is made possible through support from the Bill & Melinda Gates Foundation, FFAR and FCDO, grant OPP1172157. The authors are grateful to Lillian M LaPlace for her editorial input.
Author information
Authors and Affiliations
Contributions
All authors designed the experiments. RWK and AKR conducted the experiments. RWK and JVM wrote the manuscript and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kasili, R.W., Rai, A.K. & Moroney, J.V. LCIB functions as a carbonic anhydrase: evidence from yeast and Arabidopsis carbonic anhydrase knockout mutants. Photosynth Res 156, 193–204 (2023). https://doi.org/10.1007/s11120-023-01005-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-023-01005-1