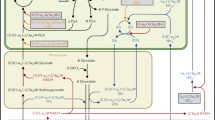
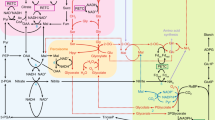
Abstract
C3 carbon fixation has a bad reputation, primarily because it is associated with photorespiration, a biochemical pathway thought to waste a substantial amount of the carbohydrate produced in a plant. This review presents evidence collected over nearly a century that (1) Rubisco when associated with Mn2+ generates additional reductant during photorespiration, (2) this reductant participates in the assimilation of nitrate into protein, and (3) this nitrate assimilation facilitates the use of a nitrogen source that other organisms tend to avoid. This phenomenon explains the continued dominance of C3 plants during the past 23 million years of low CO2 atmospheres as well as the decline in plant protein concentrations as atmospheric CO2 rises.












Similar content being viewed by others
References
Aranjuelo I, Cabrerizo PM, Arrese-Igor C, Aparicio-Tejo PM (2013) Pea plant responsiveness under elevated [CO2] is conditioned by the N source (N2 fixation versus NO3 − fertilization). Environ Exp Bot. doi:10.1016/j.envexpbot.2013.06.002
Backhausen JE et al (1998) Transgenic potato plants with altered expression levels of chloroplast NADP-malate dehydrogenase: interactions between photosynthetic electron transport and malate metabolism in leaves and in isolated intact chloroplasts. Planta 207:105–114
Backhausen JE, Kitzmann C, Horton P, Scheibe R (2000) Electron acceptors in isolated intact spinach chloroplasts act hierarchically to prevent over-reduction and competition for electrons. Photosynth Res 64:1–13
Bauwe H, Hagemann M, Fernie AR (2010) Photorespiration: players, partners and origin. Trends Plant Sci 15:330–336. doi:10.1016/j.tplants.2010.03.006
Bloom AJ, Caldwell RM, Finazzo J, Warner RL, Weissbart J (1989) Oxygen and carbon dioxide fluxes from barley shoots depend on nitrate assimilation. Plant Physiol 91:352–356
Bloom AJ, Sukrapanna SS, Warner RL (1992) Root respiration associated with ammonium and nitrate absorption and assimilation by barley. Plant Physiol 99:1294–1301
Bloom AJ, Smart DR, Nguyen DT, Searles PS (2002) Nitrogen assimilation and growth of wheat under elevated carbon dioxide. Proc Natl Acad Sci USA 99:1730–1735
Bloom AJ, Burger M, Asensio JSR, Cousins AB (2010) Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 328:899–903. doi:10.1126/science.1186440
Bloom AJ, Randall L, Taylor AR, Silk WK (2012a) Deposition of ammonium and nitrate in the roots of maize seedlings supplied with different nitrogen salts. J Exp Bot 63:1997–2006. doi:10.1093/jxb/err410
Bloom AJ, Rubio-Asensio JS, Randall L, Rachmilevitch S, Cousins AB, Carlisle EA (2012b) CO2 enrichment inhibits shoot nitrate assimilation in C3 but not C4 plants and slows growth under nitrate in C3 plants. Ecology 93:355–367
Bloom AJ, Burger M, Kimball BA, Pinter PJ (2014) Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat Clim Change 4:477–480. doi:10.1038/nclimate2183
Burnell JN (1988) The biochemistry of manganese in plants. In: Graham RD, Hannam RJ, Uren NC (eds) Manganese in soils and plants. Kluwer Academic, Dordrecht, pp 125–137
Burns IG, Zhang KF, Turner MK, Edmondson R (2010) Iso-osmotic regulation of nitrate accumulation in lettuce. J Plant Nutr 34:283–313. doi:10.1080/01904167.2011.533328
Carlisle E, Myers SS, Raboy V, Bloom AJ (2012) The effects of inorganic nitrogen form and CO2 concentration on wheat yield and nutrient accumulation and distribution. Front Plant Sci 3:195. doi:10.3389/fpls.2012.00195
Cavagnaro TR, Gleadow RM, Miller RE (2011) Plant nutrient acquisition and utilisation in a high carbon dioxide world. Funct Plant Biol 38:87–96. doi:10.1071/fp10124
Cen Y-P, Turpin DH, Layzell DB (2001) Whole-plant gas exchange and reductive biosynthesis in white lupin. Plant Physiol 126:1555–1565
Chen Z, Spreitzer RJ (1992) How various factors influence the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase/oxygenase. Photosynth Res 31:157–164
Cheng L et al (2012) Arbuscular mycorrhizal fungi increase organic carbon decomposition under elevated CO2. Science 337:1084–1087. doi:10.1126/science.1224304
Chollet R, Anderson LL (1976) Regulation of ribulose 1,5-bisphosphate carboxylase-oxygenase activities by temperature pretreatment and chloroplast metabolites. Arch Biochem Biophys 176:344–351. doi:10.1016/0003-9861(76)90173-9
Christeller JT (1981) The effects of bivalent cations on ribulose bisphosphate carboxylase/oxygenase. Biochem J 193:839–844
Christeller JT, Laing WA (1979) Effects of manganese ions and magnesium ions on the activity of soya-bean ribulose bisphosphate carboxylase/oxygenase. Biochem J 183:747–750
Chu DK, Bassham JA (1974) Activation of ribulose 1,5-diphosphate carboxylase by nicotinamide adenine-dinucleotide phosphate and other chloroplast metabolites. Plant Physiol 54:556–559. doi:10.1104/pp.54.4.556
Cleland WW, Andrews TJ, Gutteridge S, Hartman FC, Lorimer GH (1998) Mechanism of Rubisco: the carbamate as general base. Chem Rev 98:549–562. doi:10.1021/cr970010r
Collatz GJ, Berry JA, Clark JS (1998) Effects of climate and atmospheric CO2 partial pressure on the global distribution of C4 grasses: present, past, and future. Oecologia 114:441–454. doi:10.1007/s004420050468
Cotrufo MF, Ineson P, Scott A (1998) Elevated CO2 reduces the nitrogen concentration of plant tissues. Glob Change Biol 4:43–54
Cousins AB, Bloom AJ (2004) Oxygen consumption during leaf nitrate assimilation in a C3 and C4 plant: the role of mitochondrial respiration. Plant, Cell Environ 27:1537–1545
Cramer M, Myers J (1948) Nitrate reduction and assimilation in Chlorella. J Gen Physiol 32:93–102
Dortch Q (1990) The interaction between ammonium and nitrate uptake in phytoplankton. Mar Ecol Prog 61:183–201
Dukes JS et al (2005) Responses of grassland production to single and multiple global environmental changes. PLoS Biol 3:1829–1837
Dutilleul C, Lelarge C, Prioul J-L, De Paepe R, Foyer CH, Noctor G (2005) Mitochondria-driven changes in leaf NAD status exert a crucial influence on the control of nitrate assimilation and the integration of carbon and nitrogen metabolism. Plant Physiol 139:64–78
Duval BD, Blankinship JC, Dijkstra P, Hungate BA (2012) CO2 effects on plant nutrient concentration depend on plant functional group and available nitrogen: a meta-analysis. Plant Ecol 213:505–521. doi:10.1007/s11258-011-9998-8
Ehleringer JR, Cerling TE, Helliker BR (1997) C4 photosynthesis, atmospheric CO2, and climate. Oecologia 112:285–299
Ellsworth DS, Reich PB, Naumburg ES, Koch GW, Kubiske ME, Smith SD (2004) Photosynthesis, carboxylation and leaf nitrogen responses of 16 species to elevated pCO2 across four free-air CO2 enrichment experiments in forest, grassland and desert. Glob Change Biol 10:2121–2138
Epstein E, Bloom AJ (2005) Mineral nutrition of plants: principles and perspectives, 2nd edn. Sinauer Associates, Sunderland
Finzi AC et al (2007) Increases in nitrogen uptake rather than nitrogen-use efficiency support higher rates of temperate forest productivity under elevated CO2. Proc Natl Acad Sci USA 104:14014–14019
Foyer CH, Bloom AJ, Queval G, Noctor G (2009) Photorespiratory metabolism: genes, mutants, energetics, and redox signaling. Annu Rev Plant Biol 60:455–484
Frank J, Kositza MJ, Vater J, Holzwarth JF (2000) Microcalorimetric determination of the reaction enthalpy changes associated with the carboxylase and oxygenase reactions catalysed by ribulose 1,5-bisphosphate carboxylase/oxygenase (RUBISCO). Phys Chem Chem Phys 2:1301–1304
Galmes J et al (2005) Rubisco specificity factor tends to be larger in plant species from drier habitats and in species with persistent leaves. Plant, Cell Environ 28:571–579
Hall DO, Rao KK, Institute of Biology (1999) Photosynthesis. Studies in biology, 6th edn. Cambridge University Press, Cambridge
Hanson AD, Hitz WD (1983) Water deficits and the nitrogen economy. In: Taylor HM, Jordan WR, Sinclair TR (eds) Limitations to efficient water use in crop production. ASA, Madison, pp 331–343
Hodge A, Helgason T, Fitter AH (2010) Nutritional ecology of arbuscular mycorrhizal fungi. Fungal Ecol 3:267–273. doi:10.1016/j.funeco.2010.02.002
Houtz RL, Nable RO, Cheniae GM (1988) Evidence for effects on the in vivo activity of ribulose-bisphosphate carboxylase/oxygenase during development of Mn toxicity in tobacco. Plant Physiol 86:1143–1149. doi:10.1104/pp.86.4.1143
Igamberdiev AU, Bykova NV, Lea PJ, Gardestrom P (2001) The role of photorespiration in redox and energy balance of photosynthetic plant cells: a study with a barley mutant deficient in glycine decarboxylase. Physiol Plant 111:427–438
Ishijima S, Uchlbori A, Takagi H, Maki R, Ohnishi M (2003) Light-induced increase in free Mg2+ concentration in spinach chloroplasts: measurement of free Mg2+ by using a fluorescent probe and necessity of stromal alkalinization. Arch Biochem Biophys 412:126–132. doi:10.1016/s0003-9861(03)00038-9
Jordan DB, Ogren WL (1981) A sensitive assay procedure for simultaneous determination of ribulose-1,5-bisphosphate carboxylase and oxygenase activities. Plant Physiol 67:237–245
Kane HJ, Viil J, Entsch B, Paul K, Morell MK, Andrews TJ (1994) An improved method for measuring the CO2/O2 specificity of ribulosebisphosphate carboxylase-oxygenase. Funct Plant Biol 21:449–461
Kimball BA, Idso SB, Johnson S, Rillig MC (2007) Seventeen years of carbon dioxide enrichment of sour orange trees: final results. Glob Change Biol 13:2171–2183
Knaff DB (1996) Ferredoxin and ferredoxin-dependent enzymes. In: Ort DR, Yocum CF (eds) Oxygenic photosynthesis: the light reactions, vol 4., Advances in Photosynthesis. Kluwer Academic, Dordrecht, pp 333–361
Korner C (2006) Plant CO2 responses: an issue of definition, time and resource supply. New Phytol 172:393–411
Laing WA, Christeller JT (1976) A model for the kinetics of activation and catalysis of ribulose 1,5-bisphosphate carboxylase. Biochem J 159:563–570
Lekshmy S, Jain V, Khetarpal S, Pandey R (2013) Inhibition of nitrate uptake and assimilation in wheat seedlings grown under elevated CO2. Indian J Plant Physiol 18:23–29
Lilley RMC, Wang XQ, Krausz E, Andrews TJ (2003) Complete spectra of the far-red chemiluminescence of the oxygenase reaction of Mn2+-activated ribulose-bisphosphate carboxylase/oxygenase establish excited Mn2+ as the source. J Biol Chem 278:16488–16493
Long SP, Ainsworth EA, Rogers A, Ort DR (2004) Rising atmospheric carbon dioxide: plants face the future. Annu Rev Plant Biol 55:591–628
Luque-Almagro VM, Gates AJ, Moreno-Vivián C, Ferguson SJ, Richardson DJ, Roldán M (2011) Bacterial nitrate assimilation: gene distribution and regulation. Biochem Soc Trans 39:1838–1843
Matsumura H et al (2012) Crystal structure of rice Rubisco and implications for activation induced by positive effectors NADPH and 6-phosphogluconate. J Mol Biol 422:75–86
Matt P, Geiger M, Walch-Liu P, Engels C, Krapp A, Stitt M (2001) Elevated carbon dioxide increases nitrate uptake and nitrate reductase activity when tobacco is growing on nitrate, but increases ammonium uptake and inhibits nitrate reductase activity when tobacco is growing on ammonium nitrate. Plant, Cell Environ 24:1119–1137
Maynard PN, Barker AV, Minotti PL, Peck NH (1976) Nitrate accumulation in vegetables. Adv Agron 28:71–119
McCurry SD, Pierce J, Tolbert NE, Orme-Johnson WH (1981) On the mechanism of effector-mediated activation of ribulose bisphosphate carboxylase/oxygenase. J Biol Chem 256:6623–6628
McIntyre GI (1997) The role of nitrate in the osmotic and nutritional control of plant development. Aust J Plant Physiol 24:103–118
Miziorko HM, Sealy RC (1980) Characterization of the ribulosebisphosphate carboxylase-carbon dioxide-divalent cation-carboxypentitol bisphosphate complex. Biochemistry 19:1167–1171. doi:10.1021/bi00547a020
Miziorko HM, Sealy RC (1984) Electron spin resonance studies of ribulose bisphosphate carboxylase: identification of activator cation ligands. Biochemistry 23:479–485
Moroney J, Jungnick N, DiMario R, Longstreth D (2013) Photorespiration and carbon concentrating mechanisms: two adaptations to high O2, low CO2 conditions. Photosynth Res 117:121–131. doi:10.1007/s11120-013-9865-7
Maeda S-i, Konishi M, Yanagisawa S, Omata T (2014) Nitrite transport activity of a novel HPP family protein conserved in cyanobacteria and chloroplasts. Plant Cell Physiol 55:1311–1324. doi:10.1093/pcp/pcu075
Myers SS et al (2014) Increasing CO2 threatens human nutrition. Nature 510:139–142. doi:10.1038/nature13179
Norby RJ, Warren JM, Iversen CM, Medlyn BE, McMurtrie RE (2010) CO2 enhancement of forest productivity constrained by limited nitrogen availability. Proc Natl Acad Sci USA 107:19368–19373. doi:10.1073/pnas.1006463107
Ohashi Y et al (2011) Regulation of nitrate assimilation in cyanobacteria. J Exp Bot 62:1411–1424. doi:10.1093/jxb/erq427
Oliva M, Safont VS, Andres J, Tapia O (2001) Transition structures for D-ribulose-1,5-bisphosphate carboxylase/oxygenase-catalyzed oxygenation chemistry: role of carbamylated lysine in a model magnesium coordination sphere. J Phys Chem A 105:4726–4736
Osborne CP, Beerling DJ (2006) Nature’s green revolution: the remarkable evolutionary rise of C4 plants. Philos Trans R Soc B Biol Sci 361:173–194. doi:10.1098/rstb.2005.1737
Parry MAJ, Andralojc PJ, Mitchell RAC, Madgwick PJ, Keys AJ (2003) Manipulation of Rubisco: the amount, activity, function and regulation. J Exp Bot 54:1321–1333
Parry MAJ, Andralojc PJ, Scales JC, Salvucci ME, Carmo-Silva AE, Alonso H, Whitney SM (2013) Rubisco activity and regulation as targets for crop improvement. J Exp Bot 64:717–730. doi:10.1093/jxb/ers336
Pearson PN, Palmer MR (2000) Atmospheric carbon dioxide concentrations over the past 60 million years. Nature 406:695–699
Pierce J, Reddy GS (1986) The sites for catalysis and activation of ribulosebisphosphate carboxylase share a common domain. Arch Biochem Biophys 245:483–493
Pleijel H, Uddling J (2012) Yield vs. quality trade-offs for wheat in response to carbon dioxide and ozone. Glob Change Biol 18:596–605. doi:10.1111/j.1365-2486.2011.2489.x
Quesada A, Gomez-Garcia I, Fernandez E (2000) Involvement of chloroplast and mitochondria redox valves in nitrate assimilation. Trends Plant Sci 5:463–464
Rachmilevitch S, Cousins AB, Bloom AJ (2004) Nitrate assimilation in plant shoots depends on photorespiration. Proc Natl Acad Sci USA 101:11506–11510
Raghavendra AS, Carrillo NJ, Vallejos RH (1981) Differential modulation of carboxylase and oxygenase activities of ribulose 1, 5-bisphosphate carboxylase/oxygenase released from freshly ruptured spinach chloroplasts. Plant Cell Physiol 22:1113–1117
Rasse DP, Peresta G, Drake BG (2005) Seventeen years of elevated CO2 exposure in a Chesapeake Bay Wetland: sustained but contrasting responses of plant growth and CO2 uptake. Glob Change Biol 11:369–377
Rathnam CKM (1978) Malate and dihydroxyacetone phosphate-dependent nitrate reduction in spinach leaf protoplasts. Plant Physiol 62:220–223. doi:10.1104/pp.62.2.220
Reich PB et al (2006) Nitrogen limitation constrains sustainability of ecosystem response to CO2. Nature 440:922–925
Robinson JM (1987) Interactions of carbon and nitrogen metabolism in photosynthetic and non-photosynthetic tissues of higher plants: metabolic pathways and controls. In: Newman DW, Stuart KG (eds) Models in Plant Physiology and Biochemistry, vol 1. CRC Press, Boca Raton, pp 25–35
Robinson JM (1988) Spinach leaf chloroplast CO2 and NO2 − photoassimilations do not compete for photogenerated reductant manipulation of reductant levels by quantum flux density titrations. Plant Physiol 88:1373–1380
Robinson JM, Gibbs M (1982) Hydrogen peroxide synthesis in isolated spinach chloroplast lamellae: an analysis of the Mehler reaction in the presence of NADP reduction and ATP formation. Plant Physiol 70:1249–1254
Sage RF (2002) Variation in the k cat of Rubisco in C3 and C4 plants and some implications for photosynthetic performance at high and low temperature. J Exp Bot 53:609–620
Sage RF, Li M, Monson RK (1999) The taxonomic distribution of C4 photosynthesis. In: Sage RF, Monson RK (eds) C4 Plant Biology. Academic Press, San Diego, pp 551–584
Sage RF, Sage TL, Kocacinar F (2012) Photorespiration and the evolution of C4 photosynthesis. Annu Rev Plant Biol 63:19–47
Scheibe R, Dietz KJ (2012) Reduction-oxidation network for flexible adjustment of cellular metabolism in photoautotrophic cells. Plant, Cell Environ 35:202–216. doi:10.1111/j.1365-3040.2011.02319.x
Schneidereit J, Hausler RE, Fiene G, Kaiser WM, Weber APM (2006) Antisense repression reveals a crucial role of the plastidic 2-oxoglutarate/malate translocator DiT1 at the interface between carbon and nitrogen metabolism. Plant J 45:206–224
Searles PS, Bloom AJ (2003) Nitrate photoassimilation in tomato leaves under short-term exposure to elevated carbon dioxide and low oxygen. Plant, Cell Environ 26:1247–1255
Sharkey TD (1988) Estimating the rate of photorespiration in leaves. Physiol Plant 73:147–152
Skillman JB (2008) Quantum yield variation across the three pathways of photosynthesis: not yet out of the dark. J Exp Bot 59:1647–1661. doi:10.1093/Jxb/Ern029
Still CJ, Berry JA, Collatz GJ, DeFries RS (2003) Global distribution of C3 and C4 vegetation: carbon cycle implications. Glob Biogeochem Cycles 17:6-1–6-14
Studer RA, Christin P-A, Williams MA, Orengo CA (2014) Stability-activity tradeoffs constrain the adaptive evolution of RubisCO. Proc Natl Acad Sci 111:2223–2228. doi:10.1073/pnas.1310811111
Swedish Energy Agency (2003) Artificial photosynthesis. Eskilstuna, Sweden
Taiz L, Zeiger E (2010) Plant Physiology, 5th edn. Sinauer Associates, Sunderland, MA
Talhelm AF et al (2014) Elevated carbon dioxide and ozone alter productivity and ecosystem carbon content in northern temperate forests. Glob Change Biol 20:2492–2504. doi:10.1111/gcb.12564
Taniguchi M, Miyake H (2012) Redox-shuttling between chloroplast and cytosol: integration of intra-chloroplast and extra-chloroplast metabolism. Curr Opin Plant Biol 15:252–260. doi:10.1016/j.pbi.2012.01.014
Tapia O, Andrés J (1992) Towards an explanation of carboxylation/oxygenation bifunctionality in Rubisco. Transition structure for the carboxylation reaction of 2,3,4-pentanetriol. Mol Eng 2:37–41. doi:10.1007/BF00999521
Taub DR, Wang XZ (2008) Why are nitrogen concentrations in plant tissues lower under elevated CO2? A critical examination of the hypotheses. J Integr Plant Biol 50:1365–1374. doi:10.1111/j.1744-7909.2008.00754.x
Tcherkez GGB, Farquhar GD, Andrews TJ (2006) Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc Natl Acad Sci USA 103:7246–7251
Tolbert NE (1994) Role of photosynthesis and photorespiration in regulating CO2 and O2. In: Tolbert NE, Preiss J (eds) Regulation of CO2 and O2 by Photosynthetic Carbon Metabolism. Oxford University Press, New York, pp 8–33
Van Niel CB, Allen MB, Wright BE (1953) On the photochemical reduction of nitrate by algae. Biochim Biophys Acta 12:67–74. doi:10.1016/0006-3002(53)90124-3
Veen BW, Kleinendorst A (1986) The role of nitrate in osmoregulation of Italian ryegrass. Plant Soil 91:433–436. doi:10.1007/bf02198139
von Caemmerer S, Quick WP, Furbank RT (2012) The development of C4 rice: current progress and future challenges. Science 336:1671–1672
Voss I, Sunil B, Scheibe R, Raghavendra AS (2013) Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Biol 15:713–722. doi:10.1111/j.1438-8677.2012.00710.x
Walker BJ, Strand DD, Kramer DM, Cousins AB (2014) The response of cyclic electron flow around photosystem I to changes in photorespiration and nitrate assimilation. Plant Physiol 165:453–462
Warburg O, Negelein E (1920) Über die Reduktion der Salpetersäure in grünen Zellen. Biochem Z 110:66–115
Whitney SM, Houtz RL, Alonso H (2011) Advancing our understanding and capacity to engineer nature’s CO2-sequestering enzyme Rubisco. Plant Physiol 155:27–35. doi:10.1104/pp.110.164814
Wildner GF, Henkel J (1979) The effect of divalent metal ions on the activity of Mg++ depleted ribulose-1, 5-bisphosphate oxygenase. Planta 146:223–228
Wingler A, Lea PJ, Quick WP, Leegood RC (2000) Photorespiration: metabolic pathways and their role in stress protection. Philos Trans R Soc Lond Ser B Biol Sci 355:1517–1529
Zhu XG, Long SP, Ort DR (2008) What is the maximum efficiency with which photosynthesis can convert solar energy into biomass? Curr Opin Biotechnol 19:153–159
Zhu XG, Long SP, Ort DR (2010) Improving photosynthetic efficiency for greater yield. Annu Rev Plant Biol 61:235–261. doi:10.1146/annurev-arplant-042809-112206
Acknowledgments
This work was funded in part by NSF IOS-13-58675. We thank J. Clark Lagarias and George H. Lorimer for their insights about redox reactions and the reviewers for their suggestions.
Conflicts of interest
The author has no conflicts of interest with regards to this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bloom, A.J. Photorespiration and nitrate assimilation: a major intersection between plant carbon and nitrogen. Photosynth Res 123, 117–128 (2015). https://doi.org/10.1007/s11120-014-0056-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-014-0056-y