Abstract
Background and aims
The ‘home-field advantage (HFA) hypothesis’ predicts a litter-field affinity effect on litter decomposition. In terrestrial ecosystems, plant roots have comprehensive roles in regulating litter-decomposer interactions, yet their potential influences on HFA remain unsolved. To fill this gap, we conducted a litter transplant experiment in a subtropical forest, and tested whether roots affect litter-field affinity via interactions with soil microbial functions.
Methods
Leaf litters of Quercus variabilis and Pinus massoniana were incubated at their conspecific-dominated and heterospecific-dominated forests. Root-specific incubation microcosms were manipulated by using a series of root ingrowth cores to control the access of living fine roots.
Results
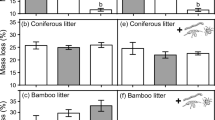
Entire exclusion of roots led to a significant suppression of HFA, and the affinity effect was amplified under a medium root constraint treatment (by 0.5 mm mesh). Incubation field (‘home’ vs. ‘away’) generally had a positive effect on litter mineralization when roots were present, and roots became more influential after 9 months than 3 months of incubation. Although microbial enzymatic functions and their impact on litter N loss depended on root status, they were not associated with incubation field.
Conclusions
Our findings advocate that a moderate amount of local roots is essential for HFA in leaf litter decomposition, and taking account of root-mediated species-specific bio-interactions will advance our understanding of native litter- home field affinity.





Similar content being viewed by others
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecology 26:32–46
Austin AT, Vivanco L, Gonzalez-Arzac A, Perez LI (2014) There's no place like home? An exploration of the mechanisms behind plant litter- decomposer affinity in terrestrial ecosystems. New Phytol 204:307–314
Ayres E, Dromph KM, Bardgett RD (2006) Do plant species encourage soil biota that specialise in the rapid decomposition of their litter? Soil Biol Biochem 38:183–186
Ayres E, Steltzer H, Simmons BL, Simpson RT, Steinweg JM, Wallenstein MD, Mellor N, Parton WJ, Moore JC, Wall DH (2009) Home-field advantage accelerates leaf litter decomposition in forests. Soil Biol Biochem 41:606–610
Badri DV, Vivanco JM (2009) Regulation and function of root exudates. Plant Cell Environ 32:666–681
Bailey VL, Peacock AD, Smith JL, Bolton H (2002) Relationships between soil microbial biomass determined by chloroform fumigation-extraction, substrate-induced respiration, and phospholipid fatty acid analysis. Soil Biol Biochem 34:1385–1389
Bais HP, Weir TL, Perry LG, Gilroy S, Vivanco JM (2006) The role of root exudates in rhizosphere interactions with plants and other organisms. Annu Rev Plant Bio 57:233–266
Berendsen RL, Pieterse CMJ, Bakker PAHM (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486
Blagodatskaya E, Kuzyakov Y (2008) Mechanisms of real and apparent priming effects and their dependence on soil microbial biomass and community structure: critical review. Biol Fertil Soils 45:115–131
Broeckling CD, Broz AK, Bergelson J, Manter DK, Vivanco JM (2008) Root exudates regulate soil fungal community composition and diversty. Appl Environ Microbiol 74:738–744
Broz AK, Manter DK, Vivanco JM (2007) Soil fungal abundance and diversity: another victim of the invasive plant Centaurea maculosa. ISME J 1:763–765
Brzostek ER, Greco A, Drake JE, Finzi AC (2013) Root carbon inputs to the rhizosphere stimulate extracellular enzyme activity and increase nitrogen availability in temperate forest soils. Biogeochemistry 115:65–76
Brzostek ER, Dragoni D, Brown ZA, Phillips RP (2015) Mycorrhizal type determines the magnitude and direction of root-induced changes in decomposition in a temperate forest. New Phytol 206:1274–1282
Butler A, Marimon BH, Maracahipes L, Marimon BS, Silvério DV, De Oliveira EA, Lenza E, Feldpauch TR, Meir P, Grace J (2013) Absorbing roots areas and transpiring leaf areas at the tropical forest and savanna boundary in Brazil In: Savannas: Climate, Biodiversity and Ecological Significance. Nova Science Publishers, Inc, pp 107–126
Caldwell BA (2005) Enzyme activities as a component of soil biodiversity: a review. Pedobiologia 49:637–644
Cesarz S, Fender AC, Beyer F, Valtanen K, Pfeiffer B, Gansert D, Hertel D, Polle A, Daniel R, Leuschner C, Scheu S (2013) Roots from beech (Fagus sylvatica L.) and ash (Fraxinus excelsior L.) differentially affect soil microorganisms and carbon dynamics. Soil Biol Biochem 61:23–32
Chen R, Senbayram M, Blagodatsky S, Myachina O, Dittert K, Lin X, Blagodatskaya E, Kuzyakov Y (2014) Soil C and N availability determine the priming effect: microbial N mining and stoichiometric decomposition theories. Glob Chang Biol 20:2356–2367
Coq S, Souquet JM, Meudec E, Cheynier V, Hättenschwiler S (2010) Interspecific variation in leaf litter tannins drives decomposition in a tropical rain forest of French Guiana. Ecology 91:2080–2091
Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VT, Godoy O, Hobbie SE, Hoorens B, Kurokawa H, Perez-Harguindeguy N, Quested HM, Santiago LS, Wardle DA, Wright IJ, Aerts R, Allison SD, van Bodegom P, Brovkin V, Chatain A, Callaghan TV, Diaz S, Garnier E, Gurvich DE, Kazakou E, Klein JA, Read J, Reich PB, Soudzilovskaia NA, Vaieretti MV, Westoby M (2008) Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol Lett 11:1065–1071
Craine JM, Morrow C, Fierer N (2007) Microbial nitrogen limitation increases decomposition. Ecology 88:2105–2113
Dick WA, Cheng L, Wang P (2000) Soil acid and alkaline phosphatase activity as pH adjustment indicators. Soil Biol Biochem 32:1915–1919
Fanin N, Fromin N, Bertrand I (2016) Functional breadth and home-field advantage generate functional differences among soil microbial decomposers. Ecology 97:1023–1037
Feng W, Liang J, Hale LE, Jung CG, Chen J, Zhou J, Xu M, Yuan M, Wu L, Bracho R, Pegoraro E, Schuur EAG, Luo Y (2017) Enhanced decomposition of stable soil organic carbon and microbial catabolic potentials by long-term field warming. Glob Chang Biol 23:4765–4776
Finzi AC, Abramoff RZ, Spiller KS, Brzostek ER, Darby BA, Kramer MA, Phillips RP (2015) Rhizosphere processes are quantitatively important components of terrestrial carbon and nutrient cycles. Glob Chang Biol 21:2082–2094
Freschet GT, Aerts R, Cornelissen JHC (2012) Multiple mechanisms for trait effects on litter decomposition: moving beyond home-field advantage with a new hypothesis. J Ecol 100:619–630
Frost PC, Benstead JP, Cross WF, Hillebrand H, Larson JH, Xenopoulos MA, Yoshida T (2006) Threshold elemental ratios of carbon and phosphorus in aquatic consumers. Ecol Lett 9:774–779
Fu SL, Cheng WX (2002) Rhizosphere priming effects on the decomposition of soil organic matter in C4 and C3 grassland soils. Plant Soil 238:289–294
Giesselmann UC, Martins KG, Brandle M, Schadler M, Marques R, Brandi R (2011) Lack of home-field advantage in the decomposition of leaf litter in the Atlantic rainforest of Brazil. Appl Soil Ecol 49:5–10
Hättenschwiler S, Coq S, Barantal S, Handa IT (2011) Leaf traits and decomposition in tropical rainforests: revisiting some commonly held views and towards a new hypothesis. New Phytol 189:950–965
Hopkins F, Gonzalez-Meler MA, Flower CE, Lynch DJ, Czimczik C, Tang JW, Subke JA (2013) Ecosystem-level controls on root-rhizosphere respiration. New Phytol 199:339–351
Hunt HW, Ingham ER, Coleman DC, Elliott ET, Reid CPP (1988) Nitrogen limitation of production and decomposition in prairie, mountain meadow, and pine Forest. Ecology 69:1009–1016
Keiser AD, Keiser DA, Strickland MS, Bradford MA (2014) Disentangling the mechanisms underlying functional differences among decomposer communities. J Ecol 102:603–609
Knorr M, Frey SD, Curtis PS (2005) Nitrogen additions and litter decomposition: a meta-analysis. Ecology 86:3252–3257
Koranda M, Schnecker J, Kaiser C, Fuchslueger L, Kitzler B, Stange CF, Sessitsch A, Zechmeister-Boltenstern S, Richter A (2011) Microbial processes and community composition in the rhizosphere of European beech - the influence of plant C exudates. Soil Biol Biochem 43:551–558
Kuzyakov Y (2010) Priming effects: interactions between living and dead organic matter. Soil Biol Biochem 42:1363–1371
Kuzyakov Y, Xu XL (2013) Competition between roots and microorganisms for nitrogen: mechanisms and ecological relevance. New Phytol 198:656–669
Kuzyakov Y, Hill PW, Jones DL (2007) Root exudate components change litter decomposition in a simulated rhizosphere depending on temperature. Plant Soil 290:293–305
Li D, Schädel C, Haddix ML, Paul EA, Conant R, Li J, Zhou J, Luo Y (2013) Differential responses of soil organic carbon fractions to warming: results from an analysis with data assimilation. Soil Biol Biochem 67:24–30
Lin H, He ZH, Hao JW, Tian K, Jia XQ, Kong XS, Akbar S, Bei ZL, Tian XJ (2017) Effect of N addition on home-field advantage of litter decomposition in subtropical forests. For Ecol Manage 398:216–225
Lindahl BD, Ihrmark K, Boberg J, Trumbore SE, Hogberg P, Stenlid J, Finlay RD (2007) Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol 173:611–620
Makkonen M, Berg MP, Handa IT, Hattenschwiler S, van Ruijven J, van Bodegom PM, Aerts R (2012) Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol Lett 15:1033–1041
Mancuso S (2012) Measuring roots: an updated approach. Springer, Berlin Heidelberg
Manzoni S, Porporato A (2009) Soil carbon and nitrogen mineralization: theory and models across scales. Soil Biol Biochem 41:1355–1379
Manzoni S, Trofymow JA, Jackson RB, Porporato A (2010) Stoichiometric controls on carbon, nitrogen, and phosphorus dynamics in decomposing litter. Ecol Monogr 80:89–106
Manzoni S, Taylor P, Richter A, Porporato A, Agren GI (2012) Environmental and stoichiometric controls on microbial carbon-use efficiency in soils. New Phytol 196:79–91
McCormack ML, Dickie IA, Eissenstat DM, Fahey TJ, Fernandez CW, Guo DL, Helmisaari HS, Hobbie EA, Iversen CM, Jackson RB, Leppalammi-Kujansuu J, Norby RJ, Phillips RP, Pregitzer KS, Pritchard SG, Rewald B, Zadworny M (2015) Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol 207:505–518
Nottingham AT, Turner BL, Winter K, Chamberlain PM, Stott A, Tanner EVJ (2013) Root and arbuscular mycorrhizal mycelial interactions with soil microorganisms in lowland tropical forest. FEMS Microbiol Ecol 85:37–50
Olson JS (1963) Energy storage and the balance of producers and decomposers in ecological systems. Ecology 44:322–331
Orwin KH, Kirschbaum MUF, St John MG, Dickie IA (2011) Organic nutrient uptake by mycorrhizal fungi enhances ecosystem carbon storage: a model-based assessment. Ecol Lett 14:493–502
Osono T, Takeda H (2002) Comparison of litter decomposing ability among diverse fungi in a cool temperate deciduous forest in Japan. Mycologia 94:421–427
Parsons SA, Congdon RA, Lawler IR (2014) Determinants of the pathways of litter chemical decomposition in a tropical region. New Phytol 203:837–882
Perez G, Aubert M, Decaens T, Trap J, Chauvat M (2013) Home-field advantage: a matter of interaction between litter biochemistry and decomposer biota. Soil Biol Biochem 67:245–254
Pfeiffer B, Fender AC, Lasota S, Hertel D, Jungkunst HF, Daniel R (2013) Leaf litter is the main driver for changes in bacterial community structures in the rhizosphere of ash and beech. Appl Soil Ecol 72:150–160
Phillips RP (2007) Towards a rhizo-centric view of plant-microbial feedbacks under elevated atmospheric CO2. New Phytol 173:664–667
Phillips RP, Meier IC, Bernhardt ES, Grandy AS, Wickings K, Finzi AC (2012) Roots and fungi accelerate carbon and nitrogen cycling in forests exposed to elevated CO2. Ecol Lett 15:1042–1049
R Development Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. R version 3.2.3. http://CRAN.R-project.org/. Accessed 7 Jan 2016
Rousk K, Michelsen A, Rousk J (2016) Microbial control of soil organic matter mineralization responses to labile carbon in subarctic climate change treatments. Glob Chang Biol 22:4150–4161
Sayer EJ, Tanner EVJ, Cheesman AW (2006) Increased litterfall changes fine root distribution in a moist tropical forest. Plant Soil 281:5–13
Schimel DS (1995) Terrestrial ecosystems and the carbon-cycle. Glob Chang Biol 1:77–91
St John MG, Orwin KH, Dickie IA (2011) No 'home' versus 'away' effects of decomposition found in a grassland-forest reciprocal litter transplant study. Soil Biol Biochem 43:1482–1489
Strickland MS, Lauber C, Fierer N, Bradford MA (2009a) Testing the functional significance of microbial community composition. Ecology 90:441–451
Strickland MS, Osburn E, Lauber C, Fierer N, Bradford MA (2009b) Litter quality is in the eye of the beholder: initial decomposition rates as a function of inoculum characteristics. Funct Ecol 23:627–636
Subke JA, Hahn V, Battipaglia G, Linder S, Buchmann N, Cotrufo MF (2004) Feedback interactions between needle litter decomposition and rhizosphere activity. Oecologia 139:551–559
Sun LJ, Kominami Y, Yoshimura K, Kitayama K (2017) Root-exudate flux variations among four co-existing canopy species in a temperate forest, Japan. Ecol Res 32:331–339
Swift MJ, Heal OW, Anderson JM (1979) Decomposition in terrestrial ecosystems. Univ of California Press, California
van der Wal A, Geydan TD, Kuyper TW, de Boer W (2013) A thready affair: linking fungal diversity and community dynamics to terrestrial decomposition processes. FEMS Microbiol Rev 37:477–494
Veen GF, Freschet GT, Ordonez A, Wardle DA (2015a) Litter quality and environmental controls of home-field advantage effects on litter decomposition. Oikos 124:187–195
Veen GF, Sundqvist MK, Wardle DA (2015b) Environmental factors and traits that drive plant litter decomposition do not determine home-field advantage effects. Funct Ecol 29:981–991
Vivanco L, Austin AT (2008) Tree species identity alters forest litter decomposition through long-term plant and soil interactions in Patagonia, Argentina. J Ecol 96:727–736
Wang FC, Fang XM, Ding ZQ, Wan SZ, Chen FS (2016) Effects of understory plant root growth into the litter layer on the leaf litter decomposition of two woody species in a subtropical forest. For Ecol Manage 364:39–45
Wickings K, Grandy AS, Reed SC, Cleveland CC (2012) The origin of litter chemical complexity during decomposition. Ecol Lett 15:1180–1188
Acknowledgements
We thank Dr. Amanda Gallinat at the Boston University and Dr. Haijing Hu at the Nanjing University for their helpful comments, assistance with English language and grammatical editing of this manuscript. This study was financially supported by the National Key Research and Development Program of the Ministry of Science and Technology of China (No. 2016YFD0600204); the State Key Program of National Natural Science Foundation of China (No. 31530007); the Sanxin Forestry Project in Jiangsu Province (No. LYSX[2016]46); the specimen platform of China and the teaching specimens sub-platform (2005DKA21403-JK); and the Major Science and Technology Program for Water Pollution Control and Treatment (No. 2012ZX07204-004-003).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Amandine Erktan
Electronic supplementary material
ESM 1
(DOCX 400 kb)
Rights and permissions
About this article
Cite this article
Tian, K., Kong, X., Gao, J. et al. Local root status: a neglected bio-factor that regulates the home-field advantage of leaf litter decomposition. Plant Soil 431, 175–189 (2018). https://doi.org/10.1007/s11104-018-3757-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-018-3757-8