Abstract
Background and aims
Accumulating a great quantity of Na+, maintaining the stability of the concentration of important nutrition elements, increasing the activities of enzymes related to ROS-scavenging are crucial strategies for the xerophyte Zygophyllum xanthoxylum surviving under adverse saline and drought environments; besides, actively regulating the photosynthesis is also a main reason for Z. xanthoxylum to adapt to mild salt conditions. However, the possible molecular basis of above physiological mechanisms is poorly understood.
Methods
By performing Illumina sequencing combined with a digital gene expression profiling technique, differentially expressed genes in leaves and roots of Z. xanthoxylum under 50 mM NaCl and −0.5 MPa osmotic stress for 6 and 24 h were identified, respectively, mainly focused on genes related to ion transport, ROS-scavenging system and photosynthesis.
Results
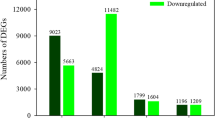
Under 50 mM NaCl and −0.5 MPa osmotic stress, the transcripts of genes encoding transporters/channels for Na+, K+, Ca2+, Mg2+, nitrogen, phosphate and important micro-elements significantly increased, which is conducive to enhance the uptake and transport of nutrient elements in Z. xanthoxylum; and more importantly, besides Na+, genes related to vacuolar compartmentalization of K+, Ca2+, NO3 − in leaves plays vital roles in the adaptation to mild salt condition. Meanwhile, NaCl treatment and osmotic stress significantly increased the transcripts of a number of genes related to ROS-scavenging system, which is beneficial to accelerate the ROS-scavenging under 50 mM NaCl and mitigate the damage of ROS to cell biomembrane system under osmotic stress. In addition, in contrast to osmotic stress, 50 mM NaCl significantly induced the expression of genes encoding proteins participated in photosynthetic electron transport and carbon fixation, while inhibited the expression of genes related to chlorophyll catabolism.
Conclusions
The present study identified potential genes underling the principal physiological mechanisms of salt and drought tolerance in Z. xanthoxylum. The results provided abundant genetic resources from desert xerophyte for genetic improvement of stress-resistance of important forage and crop species in arid area.






Similar content being viewed by others
References
Andrés Z, Pérez-Hormaeche J, Leidi EO, Schlücking K, Steinhorst L, McLachlan DH, Schumacher K, Hetherington AM, Kudla J, Cubero B, Pardo JM (2014) Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proc Natl Acad Sci U S A 111:E1806–E1814
Apse MP, Blumwald E (2007) Na+ transport in plants. FEBS Lett 581:2247–2254
Audic S, Claverie JM (1997) The significance of digital gene expression profiles. Genome Res 7:986–995
Bao AK, Wang YW, Xi JJ, Liu C, Zhang JL, Wang SM (2014) Co-expression of xerophyte Zygophyllum xanthoxylum ZxNHX and ZxVP1–1 enhances salt and drought tolerance in transgenic Lotus corniculatus by increasing cations accumulation. Funct Plant Biol 41:203–214
Bao AK, Du BQ, Touil L, Kang P, Wang QL, Wang SM (2015) Co-expression of tonoplast Cation/H+ antiporter and H+-pyrophosphatase from xerophyte Zygophyllum xanthoxylum improves alfalfa plant growth under salinity, drought, and field conditions. Plant Biotechnol J. doi:10.1111/pbi.12451
Barragán V, Leidi EO, Andrés Z, Rubio L, De Luca A, Fernández JA, Cubero B, Pardo JM (2012) Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 24:1127–1142
Bassil E, Tajima H, Liang YC, Ohto MA, Ushijima K, Nakano R, Esumi T, Coku A, Belmonte M, Blumwald E (2011) The Arabidopsis Na+/H+ antiporters NHX1 and NHX2 control vacuolar pH and K+ homeostasis to regulate growth, flower development, and reproduction. Plant Cell 23:3482–3497
Bose J, Rodrigo-Moreno A, Shabala S (2014) ROS homeostasis in halophytes in the context of salinity stress tolerance. J Exp Bot 65:1241–1257
Bräutigam A, Gowik U (2010) What can next generation sequencing do for you? Next generation sequencing as a valuable tool in plant research. Plant Biol 12:831–841
Cai JY, Ma Q, Zhou XR, Zhang JL, Wang SM (2011) Physiological role of Na+ in adaption of Zygophyllum xanthoxylum to osmotic stress. Acta Pratacul Sin 20:89–95
Cellier F, Conéjéro G, Ricaud L, Luu DT, Lepetit M, Gosti F, Casse F (2004) Characterization of AtCHX17, a member of the cation/H+ exchangers, CHX family, from Arabidopsis thaliana suggests a role in K+ homeostasis. Plant J 39:834–846
Chaves M, Flexas J, Pinheiro C (2009) Photosynthesis under drought and salt stress: regulation mechanisms from whole plant to cell. Ann Bot 103:551–560
Chen CZ, Lv XF, Li JY, Yi HY, Gong JM (2012) Arabidopsis NRT1.5 is another essential component in the regulation of nitrate reallocation and stress tolerance. Plant Physiol 159:1582–1590
Conesa A, Götz S, García-Gómez JM, Terol J, Talón M, Robles M (2005) Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21:3674–3676
Dang ZH, Zheng LL, Wang J, Gao Z, Wu SB, Qi Z, Wang YC (2013) Transcriptomic profiling of the salt-stress response in the wild recretohalophyte Reaumuria trigyna. BMC Genomics 14:29
De Angeli A, Monachello D, Ephritikhine G, Frachisse JM, Thomine S, Gambale F, Barbier-Brygoo H (2006) The nitrate/proton antiporter AtCLCa mediates nitrate accumulation in plant vacuoles. Nature 442:939–942
Deinlein U, Stephan AB, Horie T, Luo W, Xu G, Schroeder JI (2014) Plant salt-tolerance mechanisms. Trends Plant Sci 19:371–379
Dixon DP, Lapthorn A, Edwards R (2002) Plant glutathione transferases. Genome Biol 3: reviews 3004.1–10
Flowers TJ, Colmer TD (2015) Plant salt tolerance: adaptations in halophytes. Ann Bot 115:327–331
Fu HH, Luan S (1998) AtKUP1: a dual-affinity K+ transporter from Arabidopsis. Plant Cell 10:63–73
Gaxiola RA, Palmgren MG, Schumacher K (2007) Plant proton pumps. FEBS Lett 581:2204–2214
Gierth M, Mäser P (2007) Potassium transporters in plants--involvement in K+ acquisition, redistribution and homeostasis. FEBS Lett 581:2348–2356
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909–930
Gobert A, Park G, Amtmann A, Sanders D, Maathuis FJ (2006) Arabidopsis thaliana cyclic nucleotide gated channel 3 forms a nonselective ion transporter involved in germination and cation transport. J Exp Bot 57:791–800
Gobert A, Isayenkov S, Voelker C, Czempinski K, Maathuis FJ (2007) The two-pore channel TPK1 gene encodes the vacuolar K+ conductance and plays a role in K+ homeostasis. Proc Natl Acad Sci U S A 104:10726–10731
Guo Q, Wang P, Ma Q, Zhang JL, Bao AK, Wang SM (2012) Selective transport capacity for K+ over Na+ is linked to the expression levels of PtSOS1 in halophyte Puccinellia tenuiflora. Funct Plant Biol 39:1047–1057
Hauser F, Horie T (2010) A conserved primary salt tolerance mechanism mediated by HKT transporters: a mechanism for sodium exclusion and maintenance of high K(+)/Na(+) ratio in leaves during salinity stress. Plant Cell Environ 33:552–565
Hedrich R, Marten I (2011) TPC1-SV channels gain shape. Mol Plant 4:428–441
Horie T, Hauser F, Schroeder JI (2009) HKT transporter-mediated salinity resistance mechanisms in Arabidopsis and monocot crop plants. Trends Plant Sci 14:660–668
Islam MM, Munemasa S, Hossain MA, Nakamura Y, Mori IC, Murata Y (2010) Roles of AtTPC1, vacuolar two pore channel 1, in Arabidopsis stomatal closure. Plant Cell Physiol 51:302–311
Jarzyniak KM, Jasiński M (2014) Membrane transporters and drought resistance-a complex issue. Front Plant Sci 5:687
Jin YK, Jing W, Zhang Q, Zhang WH (2015) Cyclic nucleotide gated channel 10 negatively regulates salt tolerance by mediating Na+ transport in Arabidopsis. J Plant Res 128:211–220
Li XL, Borsics T, Harrington HM, Christopher DA (2005) Arabidopsis AtCNGC10 rescues potassium channel mutants of E. coli, yeast and Arabidopsis and is regulated by calcium/calmodulin and cyclic GMP in E. coli. Funct Plant Biol 32:643–653
Li YX, Ma Q, Wang SM (2014) ZxSOS1 regulates the expression of genes encoding Na+, K+ channels or transporters in the xerophyte Zygophyllum xanthoxylum under salt condition. Plant Physiol J 50:1053–1058
Lin SH, Kuo HF, Canivenc G, Lin CS, Lepetit M, Hsu PK, Tillard P, Lin HL, Wang YY, Tsai CB, Gojon A, Tsay YF (2008) Mutation of the Arabidopsis NRT1.5 nitrate transporter causes defective root-to-shoot nitrate transport. Plant Cell 20:2514–2528
Liu JQ, Pu JC, Liu XM (1987) Comparative studies on water relations and xeromorphic structures of some plant species in the middle part of the desert zone in China. Acta Bot Sin 29:662–673
Ma Q, Lou JQ, Wang SM (2010) Effect of Na+ on photosynthetic characteristics of Zygophyllum xanthoxylum seedlings under osmotic stress. Acta Pratacul Sin 19:198–203
Ma Q, Yue LJ, Zhang JL, Wu GQ, Bao AK, Wang SM (2012) Sodium chloride improves photosynthesis and water status in the succulent xerophyte Zygophyllum xanthoxylum. Tree Physiol 32:4–13
Ma Q, Li YX, Yuan HJ, Hu J, Wei L, Bao AK, Zhang JL, Wang SM (2014) ZxSOS1 is essential for long-distance transport and spatial distribution of Na+ and K+ in the xerophyte Zygophyllum xanthoxylum. Plant Soil 374:661–676
Manohar M, Shigaki T, Hirschi KD (2011) Plant cation/H+ exchangers (CAXs): biological functions and genetic manipulations. Plant Biol 13:561–569
Mardis ER (2008) The impact of next-generation sequencing technology on genetics. Trends Genet 24:133–141
Martínez J, Ledent J, Bajji M, Kinet J, Lutts S (2003) Effect of water stress on growth, Na+ and K+ accumulation and water use efficiency in relation to osmotic adjustment in two populations of Atriplex halimus L. Plant Growth Regul 41:63–73
Metzker ML (2009) Sequencing technologies-the next generation. Nat Rev Genet 11:31–46
Miller G, Shulaev V, Mittler R (2008) Reactive oxygen signaling and abiotic stress. Physiol Plant 133:481–489
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Nevo R, Charuvi D, Tsabari O, Reich Z (2012) Composition, architecture and dynamics of the photosynthetic apparatus in higher plants. Plant J 70:157–176
Pei SF, Fu H, Cen YM, Li JB (2004) Influence of Z. xanthoxylum shrubs on soil fertility in enclosure and grazing conditions. J Desert Res 24:763–767
Peiter E, Maathuis FJ, Mills LN, Knight H, Pelloux J, Hetherington AM, Sanders D (2005) The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 434:404–408
Pruzinská A, Tanner G, Anders I, Roca M, Hörtensteiner S (2003) Chlorophyll breakdown: pheophorbide a oxygenase is a Rieske-type iron-sulfur protein, encoded by the accelerated cell death 1 gene. Proc Natl Acad Sci U S A 100:15259–15264
Qiu Q, Ma T, Hu Q, Liu B, Wu Y, Zhou H, Wang Q, Wang J, Liu J (2011) Genome-scale transcriptome analysis of the desert poplar, Populus euphratica. Tree Physiol 31:452–461
Schuster SC (2008) Next-generation sequencing transforms today’s biology. Nat Methods 5:16–18
Sevilla F, Camejo D, Ortiz-Espín A, Calderón A, Lázaro JJ, Jiménez A (2015) The thioredoxin/peroxiredoxin/sulfiredoxin system: current overview on its redox function in plants and regulation by reactive oxygen and nitrogen species. J Exp Bot 66:2945–2955
Shabala S (2013) Learning from halophytes: physiological basis and strategies to improve abiotic stress tolerance in crops. Ann Bot 112:1209–1221
Sheng P, Tan J, Jin M, Wu F, Zhou K, Ma W, Heng Y, Wang J, Guo X, Zhang X, Cheng Z, Liu L, Wang C, Liu X, Wan J (2014) Albino midrib 1, encoding a putative potassium efflux antiporter, affects chloroplast development and drought tolerance in rice. Plant Cell Rep 33:1581–1594
Shi Y, Yan X, Zhao P, Yin H, Zhao X, Xiao H, Li X, Chen G, Ma XF (2013) Transcriptomic analysis of a tertiary relict plant, extreme xerophyte Reaumuria soongorica to identify genes related to drought adaptation. PLoS One 8, e63993
Song ZZ, Yang SY, Zuo J, Su YH (2014) Over-expression of ApKUP3 enhances potassium nutrition and drought tolerance in transgenic rice. Biol Plant 58:649–658
Sunarpi, Horie T, Motoda J, Kubo M, Yang H, Yoda K, Horie R, Chan WY, Leung HY, Hattori K, Konomi M, Osumi M, Yamagami M, Schroeder JI, Uozumi N (2005) Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na+ unloading from xylem vessels to xylem parenchyma cells. Plant J 44:928–938
Tang XL, Mu XM, Shao HB, Wang HY, Brestic M (2015) Global plant-responding mechanisms to salt stress: physiological and molecular levels and implications in biotechnology. Crit Rev Biotechnol 35:425–437
Tuberosa R, Salvi S (2006) Genomics-based approaches to improve drought tolerance of crops. Trends Plant Sci 11:405–412
Volkov V (2015) Salinity tolerance in plants. Quantitative approach to ion transport starting from halophytes and stepping to genetic and protein engineering for manipulating ion fluxes. Front Plant Sci 6:873
Wang SM, Wan CG, Wang YR, Chen H, Zhou ZY, Fu H, Sosebee RE (2004) The characteristics of Na+, K+ and free proline distribution in several drought-resistant plants of the Alxa Desert, China. J Arid Environ 56:525–539
Wang YJ, Yu JN, Chen T, Zhang ZG, Hao YJ, Zhang JS, Chen SY (2005) Functional analysis of a putative Ca2+ channel gene TaTPC1 from wheat. J Exp Bot 56:3051–3060
Wang P, Li Z, Wei J, Zhao Z, Sun D, Cui S (2012) A Na+/Ca2+ exchanger-like protein (AtNCL) involved in salt stress in Arabidopsis. J Biol Chem 287:44062–44070
Wu GQ, Xi JJ, Wang Q, Bao AK, Ma Q, Zhang JL, Wang SM (2011) The ZxNHX gene encoding tonoplast Na+/H+ antiporter from the xerophyte Zygophyllum xanthoxylum plays important roles in response to salt and drought. J Plant Physiol 168:758–767
Xue J, Bao YY, Li BL, Cheng YB, Peng ZY, Liu H, Xu HJ, Zhu ZR, Lou YG, Cheng JA, Zhang CX (2010) Transcriptome analysis of the brown planthopper Nilaparvata lugens. PLoS One 5:e14233
Yan K, Shao HB, Shao CY, Chen P, Zhao SJ, Brestic M, Chen XB (2013) Physiological adaptive mechanisms of plants grown in saline soil and implications for sustainable saline agriculture in coastal zone. Acta Physiol Plant 35:2867–2878
Yuan HJ, Ma Q, Wu GQ, Wang P, Hu J, Wang SM (2015) ZxNHX controls Na+ and K+ homeostasis at the whole-plant level in Zygophyllum xanthoxylum through feedback regulation of the expression of genes involved in their transport. Ann Bot 115:495–507
Yue LJ, Li SX, Ma Q, Zhou XR, Wu GQ, Bao AK, Zhang JL, Wang SM (2012) NaCl stimulates growth and alleviates water stress in the xerophyte Zygophyllum xanthoxylum. J Arid Environ 87:153–160
Zhang CJ, Zhao BC, Ge WN, Zhang YF, Song Y, Sun DY, Guo Y (2011) An apoplastic h-type thioredoxin is involved in the stress response through regulation of the apoplastic reactive oxygen species in rice. Plant Physiol 157:1884–1899
Zhou XR, Zhou ZY, Wu CX (2006) The research of the breeding characters of Zygophyllum xanthoxylum. Pratacul Sci 23:38–41
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program, grant No. 2014CB138701), the National Natural Science Foundation of China (grant Nos. 31501994, 31470503 and 31222053), Specialized Research Fund for the Doctoral Program of Higher Education of China (grant No. 20130211130001), and the Fundamental Research Funds for the Central Universities (lzujbky-2015-41).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: Frans J.M Maathuis.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
Length distribution of assembled all-Unigenes. (DOC 697 kb)
Supplementary Fig. S2
GO classification of assembled all-Unigenes. Total 43972 unigenes were assigned to at least one GO term and were grouped into 3 main GO categories and 45 sub-categories. Right Y-axis represents number of genes in a category. Left Y-axis indicates percentage of a specific category of genes in each main category. (DOC 700 kb)
Supplementary Fig. S3
COG function classification of assembled all-Unigenes. Total 26376 putative proteins showing significant homology to those in COG database were functionally classified into 25 molecular families. Y-axis indicates number of unigenes in a specific functional cluster. (DOC 700 kb)
Supplementary Table S1
Output statistics of sequencing. Total clean nucleotides is total number of clean nucleotides. Q20 percentage is proportion of nucleotides with quality value larger than 20 in reads; N percentage is proportion of unknown nucleotides in clean reads. GC percentage is proportion of guanidine and cytosine nucleotides among total nucleotides. (DOC 689 kb)
Supplementary Table S2
Summary of sequence annotation. (DOC 689 kb)
Supplementary Table S3
Differentially expressed genes (DEGs) related to ion transport in roots of Z. xanthoxylum under 50 mM NaCl for 6 h. CR6 indicates roots of plants treated as control for 6 h, SR6 indicates roots of plants treated by 50 mM NaCl for 6 h, “Fold change” equals to log2(TPM-SR6/TPM-CR6). (DOC 696 kb)
Supplementary Table S4
DEGs related to ion transport in roots of Z. xanthoxylum under −0.5 MPa osmotic stress for 6 h. CR6 indicates roots of plants treated as control for 6 h, DR6 indicates roots of plants treated by −0.5MPa osmotic stress for 6 h, “Fold change” equals to log2(TPM-DR6/TPM-CR6). (DOC 696 kb)
Supplementary Table S5
DEGs related to ion transport in leaves of Z. xanthoxylum under 50 mM NaCl for 24 h. CL24 indicates leaves of plants treated as control for 24 h, SL24 indicates leaves of plants treated by 50 mM NaCl for 24 h, “Fold change” equals to log2 (TPM-SL24/TPM-CL24). (DOC 688 kb)
Supplementary Table S6
The up-regulated DEGs related to ion transport in leaves of Z. xanthoxylum under 50 mM NaCl and −0.5 MPa osmotic stress for 24 h. (DOC 696 kb)
Supplementary Table S7
DEGs related to ROS-scavenging system in roots of Z. xanthoxylum under 50 mM NaCl for 6 h. CR6 indicates roots of plants treated as control for 6 h, SR6 indicates roots of plants treated by 50 mM NaCl for 6 h, “Fold change” equals to log2 (TPM-SR6/TPM-CR6). (DOC 693 kb)
Supplementary Table S8
DEGs related to ROS-scavenging system in roots of Z. xanthoxylum under −0.5 MPa osmotic stress for 6 h. CR6 indicates roots of plants treated as control for 6 h, DR6 indicates roots of plants treated by −0.5 MPa osmotic stress for 6 h, “Fold change” equals to log2(TPM-DR6/TPM-CR6). (DOC 696 kb)
Supplementary Table S9
DEGs related to photosynthesis in leaves of Z. xanthoxylum under 50 mM NaCl for 24 h. CL24 indicates leaves of plants treated as control for 24 h, SL24 indicates leaves of plants treated by 50 mM NaCl for 24 h, “Fold change” equals to log2 (TPM-SL24/TPM-CL24). (DOC 696 kb)
Supplementary Table S10
Expression pattern validation of selected genes under 50 mM NaCl by qPCR. The values are fold change of the expression level of each gene. Results in pPCR column represent mean standard deviations (±SD) of three experimental replicates. (DOC 692 kb)
Supplementary Table S11
Expression pattern validation of selected genes under −0.5 MPa osmotic stress by qPCR. The values are fold change of the expression level of each gene. Results in qPCR column represent mean standard deviations (±SD) of three experimental replicates. (DOC 688 kb)
Rights and permissions
About this article
Cite this article
Ma, Q., Bao, AK., Chai, WW. et al. Transcriptomic analysis of the succulent xerophyte Zygophyllum xanthoxylum in response to salt treatment and osmotic stress. Plant Soil 402, 343–361 (2016). https://doi.org/10.1007/s11104-016-2809-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-016-2809-1