Abstract
Aims
Nitrous oxide (N2O) is a strong greenhouse effective gas (GHG); the primary human source of N2O is agricultural activities. Excessive nitrogen (N) fertilization of agricultural land is now widely recognized as a major contributor. In soil, the microbial processes of nitrification and denitrification are the principal sources of N2O. However, it remains poorly understood how conventional hydroponics influences GHG emission. Here, we compared GHG fluxes from soil and rockwool used for hydroponics under identical nutrient conditions.
Methods
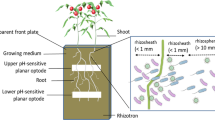
Tomato plants (Solanum lycopersicum, momotaro) were grown in soil or by hydroponics using rockwool. In situ emissions of CH4, CO2, and N2O, and the abundance of genes involved in nitrification and denitrification were measured during cultivation.
Results
Hydroponics with rockwool mitigated CO2 emission by decreasing the microbial quantity in the rhizosphere. Dilution of the nutrient solution significantly decreased N2O emission from rockwool. Although proliferation of nitrifiers or denitrifiers in the rhizosphere did not induce N2O emission, reuse or long-term use of rockwool induced a 3.8-fold increase in N2O emission.
Conclusions
Our data suggest that hydroponics has a lower environmental impact and that adequate fertilizer application, rather than bacterial control, governs N2O fluxes in hydroponic cultivation using rockwool.





Similar content being viewed by others
References
Acton SD, Baggs EM (2011) Interactions between N application rate, CH4 oxidation and N2O production in soil. Biogeochemistry 103:15–26
Ahn JH, Kim MC, Shin HC, Choi MK, Yoon SS, Kim T, Song HG, Lee GH, Ka JO (2006) Improvement of PCR amplification bias for community structure analysis of soil bacteria by denaturing gradient gel electrophoresis. J Microbiol Biotechnol 16:1561–1569
Ahn JH, Kim S, Park H, Rahm B, Pagilla K, Chandran K (2010) N2O emissions from activated sludge processes, 2008–2009: results of a national monitoring survey in the United States. Environ Sci Technol 44:4505–4511
Ahn JH, Kwan T, Chandran K (2011) Comparison of partial and full nitrification processes applied for treating high-strength nitrogen wastewaters: microbial ecology through nitrous oxide production. Environ Sci Technol 45:2734–2740
Alam MS, Jia Z (2012) Inhibition of methane oxidation by nitrougenous fertilizers in a paddy soil. Front Microbial 3:1–13
Anton A, Montero JI, Munoz P, Castells F (2005) LCA and tomato production in Mediterranean greenhouses. Int J Agr Res Gov Eco 4:102–112
Berendsen RL, Pieterse CM, Bakker PA (2012) The rhizosphere microbiome and plant health. Trends Plant Sci 17:478–486
Bergsma TT, Robertson GP, Ostrom NE (2002) Influence of soil moisture and land use history on denitrification end-products. J Environ Qual 31:711–717
Braker G, Fesefeldt A, Witzel KP (1998) Development of PCR primer systems for amplification of nitrite reductase genes (nirK and nirS) to detect denitrifying bacteria in environmental samples. Appl Environ Microbiol 64:3769–3775
Daum D, Schenk MK (1996) Gaseous nitrogen losses from a soilless culture system in the greenhouse. Plant soil 183:69–78
Degrange V, Bardin R (1995) Detection and counting of Nitrobacter populations in soil by PCR. Appl Environ Microbiol 61:2093–2098
Dionisi HM, Layton AC, Harms G, Gregory IR, Robinson KG, Sayler GS (2002) Quantification of Nitrosomonas oligotropha-like ammonia-oxidizing bacteria and Nitrospira spp. from full-scale wastewater treatment plants by competitive PCR. Appl Environ Microbiol 68:245–253
Duineveld BM, Rosado AS, Van Elsas JD, Van Veen JA (1998) Analysis of the dynamics of bacterial communities in the rhizosphere of the Chrysanthemum via denaturing gradient gel electrophoresis and substrate utilization patterns. Appl Environ Microbiol 64:4950–4957
Eichner MJ (1990) Nitrous oxide emissions from fertilized soils: summary of available data. J Environ Quality 19:272–280
Hwang S, Hanaki K (2000) Effects of oxygen concentration and moisture content of refuse on nitrification, denitrification, and nitrous oxide production. Bioresour Technol 71:159–169
Ibekwe AM, Poss JA, Grattan SR, Grieve CM, Suarez D (2010) Bacterial diversity in cucumber (Cucumis sativus) rhizosphere in response to salinity, soil pH, and boron. Soil Biol Biochem 42:567–575
Inamori R, Wang Y, Yamamoto T, Zhang J, Kong H, Xu K, Inamori Y (2008) Seasonal effect on N2O formation in nitrification in constructed wetlands. Chemosphere 73:1071–1077
Ingram DL, Thomas Fernandez R (2012) Life cycle assessment: a tool for determining the environmental impact of horticultural crop production. Hort Technology 22:275–279
IPCC (2001) The supplementary report to the IPCC scientific assessment. Cambridge University Press, Cambridge
IPCC (2007) Climate change. Cambridge University Press, Cambridge, UK
Itakura M, Tabata K, Eda S, Mitsui H, Murakami K, Yasuda J, Minamisawa K (2008) Generation of Bradyrhizobium japonicum mutants with increased N2O reductase activity by selection after introduction of a mutated dnaQ gene. Appl Environ Microbiol 74:7258–7264
Itakura M, Uchida Y, Akiyama H, Takada-Hoshino Y, Shimomura Y, Morimoto S, Tago K, Wang Y, Hayakawa C, Uetake Y, Sanchez C, Eda S, Hayatsu M, Minamisawa K (2012) Mitigation of nitrous oxide emissions from soils by Bradyrhizobium japonicum inoculation. Nature clinate change 11:1–5
Kandeler E, Deiglmayr K, Tscherko D, Bru D, Philippot L (2006) Abundance of narG, nirS, nirK, and nosZ genes of denitrifying bacteria during primary successions of a glacier foreland. Appl Environ Microbiol 72:5957–5962
Kennedy TL, Suddick EC, Six J (2013) Reduced nitrous oxide emissions and increased yields in California tomato cropping systems under drip irrigation and fertigation. Agric Ecosyst Environ 170:16–27
Kool DM, Dolfing J, Wrage N, Van Groenigen JW (2011) Nitrifier denitrification as a distinct and significant source of nitrous oxide from soil. Soil Biol Biochem 43:174–178
Kowalchuk GA, Stephen JR, de Boer W, Prosser JI, Embley TM, Woldendorp JW (1997) Analyses of ammonia-oxidizing bacteria of the β subdivision of the class Proteobacteria in coastal sand dunes bydenaturing gradient gel electrophoresis and sequencing of PCR-amplified 16S ribosomal DNA fragments. Appl Environ Microbial 63:1489–1497
Li K, Gong Y, Song W, He G, Hu Y, Tian C, Liu X (2012) Responses of CH(4), CO(2) and N(2)O fluxes to increasing nitrogen deposition in alpine grassland of the Tianshan Mountains. Chemosphere 88:140–143
Liu X, Chen CR, Wang WJ, Hughes JM, Lewis T, Hou EQ, Shen J (2013) Soil environmental factors rather than denitrification gene abundance control N2O fluxes in a wet scerophyll forest with different burning frequency. Soil Biol Biochem 57:292–300
Nkongolo NV, Hatano R, Kakembo V (2010) Diffusivity models and greenhouse gases fluzes from a forest, pasture, grasslans and corn field in northern Hokkaido, Japan. Pedosphere 20:747–760
Penton CR, Deenik JL, Popp BN, Bruland GL, Engstrom P, Louis DS, Tiedje J (2013) Importance of sub-surface rhizosphere-mediated coupled nitrification-denitrification in a flooded agroecosystem in Hawaoo. Soil Biol Biochem 57:362–373
Pluimers JC, Kroeze C, Bakker EJ, Challa H, Hordijk L (2000) Quantifying the environmental impact of production in agriculture and horticulture in The Netherlands: which emissions do we need to consider? Agric Syst 66:167–189
Postma J, Geraats BPJ, Pastoor R, van Elsas JD (2005) Characterization of the microbial community involved in the suppression of Pythium aphanidermatum in cucumber grown on rockwool. Phytophathology 95:808–818
Robertson GP, Paul EA, Harwood RR (2000) Greenhouse gases in intensive agriculture: contributions of individual gases to the radiative forcing of the atmosphere. Science 289:1922–1925
Rolston DE (1986) Method of Soil Analysis, Part 1, Physical and Mineralogical Methods, 2nd ed., Agronomy Monograph. pp. 1103–1119
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: molecular fine-scale analysis of natural ammonia-oxidizing populations. Appl Environ Microbiol 63:4704–4712
Ryu DK, Chung SO, Park SU, Kim SJ, Lim YP (2012) Construction and operatinoal test of a plant factory. In American Society of Agricultural and Biological Engineers Annual International Meeting 2012:5497–5505
Throback IN, Enwall K, Jarvis A, Hallin S (2004) Reassessing PCR primers targeting nirS, nirK and nosZ genes for community surveys of denitrifying bacteria with DGGE. FEMS Microbiol Ecol 49:401–417
Tiedje JM (1988) Ecology of denitrification and dissimilatory nitrate reduction to ammonium. John Wiley & Sons, New York
Torrellas M, Anton A, Lopez JC, Baeza EJ, Parra JP, Munoz P, Montero JI (2012) LCA of a tomato crop in a multi-tunnel greenhouse in Almeria. Int J Life Cycle Assess 17:863–875
Torrellas M, Anton A, Montero JI (2013) An environmental impact calculator for greenhouse production systems. J Environ Manage 118:186–195
Van't Oster A, Van Ieperen W, Kalaitzoglou P (2012) Model study on applicability of a semi closed greenhouse concept in Almeria: effects on greenhouse climate, crop yield and resource use efficiency. Acta Horticulture 927:51–58
Wang WJ, Dalal RC, Moody PW, Smith CJ (2003) Relationship of soil respiration and microbial biomass, substrate availability and clay content. Soil Biol Biochem 35:273–284
Wang Y, Inamori R, Kong H, Xu K, Inamori Y, Kondo T, Zhang J (2008) Nitrous oxide emission from polyculture constructed wetlands: effect of plant species. Environ Pollut 152:351–360
Acknowledgments
We thank Mr. Hiroshi Shimura, Ms. Miki Ueda, and Ms. Mari Sato (Co. Ltd. Ceres) for their technical assistance in the cultivation, sampling and gas analyses, and helpful comments on the manuscript. This work was supported by Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research to T. Y., F. G., K. S. and S.-n.H., Grant Number 22380139.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Liz Shaw.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 23 kb)
Rights and permissions
About this article
Cite this article
Hashida, Sn., Johkan, M., Kitazaki, K. et al. Management of nitrogen fertilizer application, rather than functional gene abundance, governs nitrous oxide fluxes in hydroponics with rockwool. Plant Soil 374, 715–725 (2014). https://doi.org/10.1007/s11104-013-1917-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1917-4