Abstract
Background & aims
Herbivore-driven changes to soil properties can influence the decomposition rate of organic material and therefore soil carbon cycling within grassland ecosystems. We investigated how aboveground foraging mammalian and invertebrate herbivores affect mineral soil decomposition rates and associated soil properties in two subalpine vegetation types (short-grass and tall-grass) with different grazing histories.
Methods
Using exclosures with differing mesh sizes, we progressively excluded large, medium and small mammals and invertebrates from the two vegetation types in the Swiss National Park (SNP). Mineral soil decomposition rates were assessed using the cotton cloth (standard substrate) method between May and September 2010.
Results
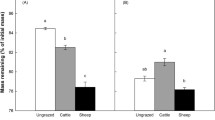
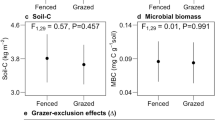
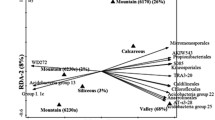
Decomposition displayed strong spatio-temporal variability, best explained by soil temperature. Exclusion of large mammals increased decomposition rates, but further exclusion reduced decomposition rates again in the lightly grazed (tall-grass) vegetation. No difference among treatments was found in the heavily grazed (short-grass) vegetation. Heavily grazed areas had higher decomposition rates than the lightly grazed areas because of higher soil temperatures. Microbial biomass carbon and soil C:N ratio were also linked to spatio-temporal decomposition patterns, but not to grazing history.
Conclusions
Despite altering some of the environmental controls of decomposition, cellulose decomposition rates in the SNP’s subalpine grasslands appear to be mostly resistant to short-term herbivore exclusion.




Similar content being viewed by others
Abbreviations
- SNP:
-
Swiss National Park
- C:
-
Carbon
- N:
-
Nitrogen
- P:
-
Phosphorus
- OM:
-
Organic matter
- MBC:
-
Microbial biomass carbon
- PCA:
-
Principal component analysis
- PC:
-
Principal component
- PWP:
-
Permanent wilting point
- CTSL:
-
Cotton tensile strength loss
- CRR:
-
Cotton rotting rate
- q mic :
-
Microbial quotient (microbial C:soil C)
References
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Aerts R, De Caluwe H, Beltman B (2003) Plant community mediated vs. nutritional controls on litter decomposition rates in grasslands. Ecology 84:3198–3208
Anderson JPE, Domsch KH (1978) A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol Biochem 10:215–221
Anderson T-H, Domsch K (1989) Ratios of microbial biomass carbon to total organic carbon in arable soils. Soil Biol Biochem 21:471–479
Anderson JM, Hetherington SL (1999) Temperature, nitrogen availability and mixture effects on the decomposition of heather [Calluna vulgaris (L.) Hull] and bracken [Pteridium aquilinum (L.) Kuhn] litters. Funct Ecol 13:116–124
Archer S, Detling JK (1986) Evaluation of potential herbivore mediation of plant water status in a North-American mixed-grass prairie. Oikos 47:287–291
Bakker ES, Olff H, Boekhoff M, Gleichman JM, Berendse F (2004) Impact of herbivores on nitrogen cycling: contrasting effects of small and large species. Oecologia 138:91–101
Bardgett RD, Wardle DA (2003) Herbivore-mediated linkages between aboveground and belowground communities. Ecology 84:2258–2268
Conant RT, Ryan MG, Ågren GI, Birge HE, Davidson EA, Eliasson PE, Evans SE, Frey SD, Giardina CP, Hopkins FM, Hyvönen R, Kirschbaum MUF, Lavallee JM, Leifeld J, Parton WJ, Steinweg JM, Wallenstein MD, Wetterstedt JÅM, Bradford MA (2011) Temperature and soil organic matter decomposition rates—synthesis of current knowledge and a way forward. Glob Chang Biol 17:3392–3404
Cornelissen JHC, van Bodegom PM, Aerts R, Callaghan TV, van Logtestijn RSP, Alatalo J, Chapin FS, Gerdol R, Gudmundsson J, Gwynn-Jones D, Hartley AE, Hik DS, Hofgaard A, Jonsdottir IS, Karlsson S, Klein JA, Laundre J, Magnusson B, Michelsen A, Molau U, Onipchenko VG, Quested HM, Sandvik SM, Schmidt IK, Shaver GR, Solheim B, Soudzilovskaia NA, Stenstrom A, Tolvanen A, Totland O, Wada N, Welker JM, Zhao XQ, Team MOL (2007) Global negative vegetation feedback to climate warming responses of leaf litter decomposition rates in cold biomes. Ecol Lett 10:619–627
Drewnik M (2006) The effect of environmental conditions on the decomposition rate of cellulose in mountain soils. Geoderma 132:116–130
Epstein HE, Burke IC, Lauenroth WK (2002) Regional patterns of decomposition and primary production rates in the U.S. Great Plains. Ecology 83:320–327
Fisk MC, Schmidt SK, Seastedt TR (1998) Topographic patterns of above- and belowground production and nitrogen cycling in Alpine tundra. Ecology 79:2253–2266
Fog K (1988) The effect of added nitrogen on the rate of decomposition of organic matter. Biol Rev 63:433–462
French D (1988) Seasonal patterns in cotton strip decomposition in soils. In: Harrison AF, Latter PM, Walton DWH (eds) Cotton strip assay: an index for decomposition in soils. Institute of Terrestrial Ecology, Grange-Over-Sands, pp 46–49
Gardiner T, Pye M, Field R, Hill J (2002) The influence of sward height and vegetation composition in determining the habitat preferences of three Chorthippus species (Orthoptera: Acrididae) in Chelmsford, Essex, UK. J Orthoptera Res 11:207–213
Gavrichkova O, Moscatelli MC, Kuzyakov Y, Grego S, Valentini R (2010) Influence of defoliation on CO2 efflux from soil and microbial activity in a Mediterranean grassland. Agric Ecosyst Environ 136:87–96
Gee GW, Bauder JW (1986) Particle-size analysis. In: Klute A (ed) Methods of soil analysis part 1. Physical and mineralogical methods, 2nd edn. ASA-SSSA, Madison, pp 383–412
Güsewell S, Jewell PL, Edwards PJ (2005) Effects of heterogeneous habitat use by cattle on nutrient availability and litter decomposition in soils of an Alpine pasture. Plant Soil 268:135–149
Gutknecht JLM, Field CB, Balser TC (2012) Microbial communities and their responses to simulated global change fluctuate greatly over multiple years. Glob Chang Biol 18:2256–2269
Hafner S, Unteregelsbacher S, Seeber E, Lena B, Xu X, Li X, Guggenberger G, Miehe G, Kuzyakov Y (2012) Effect of grazing on carbon stocks and assimilate partitioning in a Tibetan montane pasture revealed by 13CO2 pulse labeling. Glob Chang Biol 18:528–538
Hamilton EW, Frank DA, Hinchey PM, Murray TR (2008) Defoliation induces root exudation and triggers positive rhizospheric feedbacks in a temperate grassland. Soil Biol Biochem 40:2865–2873
Harmon ME, Franklin JF, Swanson FJ, Sollins P, Gregory SV, Lattin JD, Anderson NH, Cline SP, Aumen NG, Sedell JR, Lienkaemper GW, Cromack K Jr, Cummins KW (1986) MacFadyen, A & Ford, E (eds) Ecology of coarse woody debris in temperate ecosystems. Adv Ecol Res 15:133–302
Hill MO, Latter PM, Bancroft G (1985) A standard curve for inter-site comparison of cellulose degradation using the cotton strip method. Can J Soil Sci 65:609–619
Hobbie SE (2000) Interactions between litter lignin and soil nitrogen availability during leaf litter decomposition in a Hawaiian montane forest. Ecosystems 3:484–494
Hodel M (2011) Top-down effects of different sized herbivores on soil microbial biomass C and bacterial community structure in subalpine grasslands. MSc. Thesis, Institute of Evolutionary Biology and Environmental Sciences, University of Zürich
Ineson P, Bacon P, Lindley D (1988) Decomposition of cotton strips in soil: analysis of the world data set. In: Harrison AF, Latter PM, Walton DWH (eds) Cotton strip assay: an index for decomposition in soils. Institute of Terrestrial Ecology, Grange-Over-Sands, pp 155–165
Klute A (1986) Water retention. In: Klute A (ed) Methods of soil analysis part 1. Physical and mineralogical methods, 2nd edn. ASA-SSSA, Madison, pp 635–662
Knapp AK, Blair JM, Briggs JM, Collins SL, Hartnett DC, Johnson LC, Towne EG (1999) The keystone role of bison in north American tallgrass prairie—Bison increase habitat heterogeneity and alter a broad array of plant, community, and ecosystem processes. Bioscience 49:39–50
Kuo S (1996) Phosphorus. In: Sparks DL (ed) Methods of soil analysis: part 3- chemical methods. ASA-SSSA, Madison, pp 869–919
Latter PM, Harrison AF (1988) Decomposition of cellulose in relation to soil properties and plant growth. In: Harrison AF, Latter PM, Walton DWH (eds) Cotton strip assay: an index for decomposition in soils. Institute of Terrestrial Ecology, Grange-Over-Sands, pp 68–71
Latter PM, Howson G (1977) The use of cotton strips to indicate cellulose decomposition in the field. Pedobiologia 17:145–155
Latter PM, Walton D (1988) The cotton strip assay for cellulose decomposition studies in soil: history of the assay and development. In: Harrison AF, Latter PM, Walton DWH (eds) Cotton strip assay: an index for decomposition in soils. Institute of Terrestrial Ecology, Grange-Over-Sands, pp 7–10
McNaughton SJ (1979) Grazing as an optimization process: grass-ungulate relationships in the Serengeti. Am Nat 113:691–703
MeteoSchweiz (2011) IDAWEB weather data portal. https://gate.meteoswiss.ch/idaweb/login.do?language=en. Accessed 10 Jan 2011
Neff DJ (1968) The pellet-group count technique for big game trends, census, and distribution: a review. J Wildl Manag 32:597–614
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Wagner H (2011) vegan: Community Ecology Package. R package version 2.0–1. http://CRAN.R-project.org/package=vegan
Pastor J, Naiman RJ, Dewey B, McInnes P (1988) Moose, microbes, and the boreal forest. Bioscience 38:770–777
Paterson E, Thornton B, Sim A, Pratt S (2003) Effects of defoliation and atmospheric CO2 depletion on nitrate acquisition, and exudation of organic compounds by roots of Festuca rubra. Plant Soil 250:293–305
Piñeiro G, Paruelo JM, Oesterheld M, Jobbagy EG (2010) Pathways of grazing effects on soil organic carbon and nitrogen. Rangel Ecol Manag 63:109–119
Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Developement Team (2011) nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–102. http://CRAN.R-project.org/package=nlme
Plante AF, Parton WJ (2007) The dynamics of soil organic matter and nutrient cycling. In: Paul EA (ed) Soil microbiology, ecology and biochemistry, 3rd edn. Elsevier, Oxford, pp 433–464
R Development Core Team (2011) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Risch AC, Jurgensen MF, Frank DA (2007) Effects of grazing and soil micro-climate on decomposition rates in a spatio-temporally heterogeneous grassland. Plant Soil 298:191–201
Risch AC, Haynes AG, Busse MD, Filli F, Schütz M (2013) The response of soil CO2 fluxes to progressively excluding vertebrate and invertebrate herbivores depends on ecosystem type. Ecosystems. doi:10.1007/s10021-013-9676-x
Robinson C (2002) Controls on decomposition and soil nitrogen availability at high latitudes. Plant Soil 242:65–81
Robinson CH, Wookey PA, Parsons AN, Potter JA, Callaghan TV, Lee JA, Press MC, Welker JM (1995) Responses of plant litter decomposition and nitrogen mineralisation to simulated environmental change in a high arctic polar semi-desert and a subarctic dwarf shrub heath. Oikos 74:503–512
Rossignol N, Bonis A, Bouzille J-B (2011) Grazing-induced vegetation patchiness controls net N mineralization rate in a semi-natural grassland. Acta Oecol Int J Ecol 37:290–297
Schmidt E, Ruschmeyer O (1958) Cellulose decomposition in soil burial beds I. Soil properties in relation to cellulose degradation. Appl Phys Lett 6:108–114
Schmidt MWI, Torn MS, Abiven S, Dittmar T, Guggenberger G, Janssens IA, Kleber M, Kögel-Knabner I, Lehmann J, Manning DAC, Nannipieri P, Rasse DP, Weiner S, Trumbore SE (2011) Persistence of soil organic matter as an ecosystem property. Nature 478:49–56
Schütz M, Risch AC, Achermann G, Thiel-Egenter C, Page-Dumroese DS, Jurgensen MF, Edwards PJ (2006) Phosphorus translocation by red deer on a subalpine grassland in the Central European Alps. Ecosystems 9:624–633
Shand C, Macklon A, Edwards A, Smith S (1994) Inorganic and organic P in soil solutions from three upland soils. Plant Soil 160:161–170
Shariff AR, Biondini ME, Grygiel CE (1994) Grazing intensity effects on litter decomposition and soil-nitrogen mineralization. J Range Manag 47:444–449
Singer FJ, Harter MK (1996) Comparative effects of elk herbivory and 1988 fires on northern Yellowstone National Park grasslands. Ecol Appl 6:185–199
Sistla SA, Asao S, Schimel JP (2012) Detecting microbial N-limitation in tussock tundra soil: implications for Arctic soil organic carbon cycling. Soil Biol Biochem 55:78–84
Sowerby A, Blum H, Gray TRG, Ball AS (2000) The decomposition of Lolium perenne in soils exposed to elevated CO2: comparisons of mass loss of litter with soil respiration and soil microbial biomass. Soil Biol Biochem 32:1359–1366
Spalinger LC, Haynes AG, Schütz M, Risch AC (2012) Impact of wild ungulate grazing on Orthoptera abundance and diversity in subalpine grasslands. Insect Conserv Divers 5:444–452
Thiel-Egenter C, Risch AC, Jurgensen MF, Page-Dumroese DS, Krusi BO, Schütz M (2007) Response of a subalpine grassland to simulated grazing: aboveground productivity along soil phosphorus gradients. Community Ecol 8:111–117
Wardle DA, Bonner KI, Barker GM (2002) Linkages between plant litter decomposition, litter quality, and vegetation responses to herbivores. Funct Ecol 16:585–595
Wardle DA, Bardgett RD, Klironomos JN, Setala H, van der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633
Wesche K, Ronnenberg K, Retzer V, Miehe G (2010) Effects of large herbivore exclusion on southern Mongolian desert steppes. Acta Oecol 36:234–241
White R, Murray S, Rohweder M (2000) Pilot analysis of global ecosystems: grassland ecosystems technical report. World Resources Institute, Washington, DC
Withington CL, Sanford RL Jr (2007) Decomposition rates of buried substrates increase with altitude in the forest-alpine tundra ecotone. Soil Biol Biochem 39:68–75
Acknowledgments
We would like to thank the employees and volunteers of the Swiss Federal Institute for Forest, Snow and Landscape Research and the Swiss National Park for assistance with fence construction, data collection and lab analyses. Special thanks go to Anna Schweiger for assistance preparing the cloths. We are also grateful to the SNP for administrative support throughout our research, as well as Douglas A. Frank and three anonymous reviewers for constructive comments on previous versions of the manuscript. This study was funded by the Swiss National Science Foundation, SNF grant-no 31003A_122009/1, to ACR, MS and Flurin Filli (SNP).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Katja Klumpp.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 90 kb)
Rights and permissions
About this article
Cite this article
Haynes, A.G., Schütz, M., Buchmann, N. et al. Linkages between grazing history and herbivore exclusion on decomposition rates in mineral soils of subalpine grasslands. Plant Soil 374, 579–591 (2014). https://doi.org/10.1007/s11104-013-1905-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-013-1905-8