Abstract
Background and aims
Roots of the lowest branch orders have the highest mortality rate, and may contribute predominately to plant carbon (C) and nutrient transfer into the soil. Yet patterns and controlling factors of the decomposition of these roots are poorly understood.
Methods
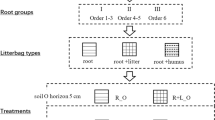
We conducted a two-year field litterbag study on different root orders and leaf litter in four temperate and four subtropical tree species.
Results
Five species showed slower decay rates in lower- (order 1–2) than higher-order (order 3–5) roots, and all species showed slower decay rates in lower-order roots than leaf litter. These patterns were strongly related to higher acid-insoluble fraction in lower- than higher-order roots, and in roots than in leaf litter, but were unrelated to initial N concentration. Litter N was predominantly in recalcitrant forms and limited amount of N was released during the study period;only 12 % of root N and 26 % of leaf litter N was released in 2 years.
Conclusions
We conclude that the slow decomposition of lower-order roots may be a common phenomenon and is mainly driven by their high acid-insoluble fraction. Moreover, litter N, especially root N, is retained during decomposition and may not be available for immediate plant uptake.




Similar content being viewed by others
References
Aerts R (1997) Climate, leaf litter chemistry and leaf litter decomposition in terrestrial ecosystems: a triangular relationship. Oikos 79:439–449
Berg B, McClaugherty C (2008) Plant litter–eecomposition, humus formation, carbon sequestration (2nd edition). Springer-Verlag Berlin, Heidelberg, Germany
Bird JA, Kleber M, Torn MS (2008) 13C and 15N stabilization dynamics in soil organic matter fractions during needle and fine root decomposition. Org Geochem 39:465–477
Christenson LM, Lovett GM, Mitchell MJ, Groffman PM (2002) The fate of nitrogen in Gypsy moth frass deposited to an oak forest floor. Oecologia 131:444–452
Coley PD, Bryant JP, Chapin FS III (1985) Resource availability and plant antiherbivore defense. Science 230:895–899
Crow SE, Lajtha K, Filley TR, Swanston CW, Bowden RD, Caldwell BA (2009) Sources of plant-derived carbon and stability of organic matter in soil: implications for global change. Global Change Biol 15:2003–2019
Davidson EA, Janssens IA (2006) Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 440:165–173
Dornbush ME, Isenhart TM, Raich JW (2002) Quantifying fine-root decomposition: an alternative to buried litterbags. Ecology 83:2985–2990
Fan P, Guo D (2010) Slow decomposition of lower order roots: a key mechanism of root carbon and nutrient retention in the soil. Oecologia 163:509–515
Fernandez CW, Koide RT (2012) The role of chitin in the decomposition of ectomycorrhizal fungal litter. Ecology 93:24–28
Gessner MO, Neumann PTM (2005) Total lipids. In: Graca MAS, Bärlocher F, Gessner MO (eds) Methods to study litter decomposition: a practical guide. Springer, Dordrecht, The Netherlands, pp 91–95
Gholz HL, Wedin DA, Smitherman SM, Harmon ME, Parton WJ (2000) Long-term dynamics of pine and hardwood litter in contrasting environments: toward a global model of decomposition. Global Change Biol 6:751–765
Goebel M, Hobbie SE, Bulaj B, Zadworny M, Archibald DD, Oleksyn J, Reich PB, Eissenstat DM (2011) Decomposition of the finest root branching orders: linking belowground dynamics to fine-root function and structure. Ecol Monog 81:89–102
Gonzalez G, Seastedt TR (2001) Soil fauna and plant litter decomposition in tropical and subalpine forests. Ecology 82:955–964
Guo DL, Mitchell RJ, Hendricks JJ (2004) Fine root branch orders respond differentially to carbon source-sink manipulations in a longleaf pine forest. Oecologia 140:450–457
Guo DL, Xia M, Wei X, Chang W, Shi W, Wang Z (2008a) Anatomical traits associated with absorption and mycorrhizal colonization are linked to root branch order in twenty-three Chinese temperate tree species. New Phytol 180:673–683
Guo DL, Mitchell RJ, Withington JM, Fan PP, Hendricks JJ (2008b) Endogenous and exogenous controls of root life span, mortality and nitrogen flux in a longleaf pine forest: root branch order predominates. J Ecol 96:737–745
Hättenschwiler S, Bracht Jørgensen H (2010) Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J Ecol 98:754–763
Hendricks JJ, Aber JD, NadelhoVer KJ, Hallett RD (2000) Nitrogen controls on fine root substrate quality in temperate forest ecosystems. Ecosystems 3:57–69
Jackson RB, Mooney HA, Schulze E-D (1997) A global budget for fine root biomass, surface area, and nutrient contents. PNAS 94:7362–7366
Kätterer T, Anders Bolinder M, Andren O, Kirchmann H, Menichetti L (2011) Roots contribute more to refractory soil organic matter than above-ground crop residues, as revealed by a long-term field experiment. Agric Ecosyst Environ 141:184–192
Klopatek JM (2007) Litterfall and fine root biomass contribution to nutrient dynamics in second- and old-growth Douglas-fir ecosystems. Plant Soil 294:157–167
Langley JA, Chapman SK, Hungate BA (2006) Ectomycorrhizal colonization slows root decomposition: the post-mortem fungal legacy. Ecol Lett 9:955–959
Li Z, Peng S, Rae DJ, Zhou G (2001) Litter decomposition and nitrogen mineralization of soils in subtropical plantation forests of southern China, with special attention to comparisons between legumes and non-legumes. Plant Soil 229:105–116
Lindahl BD, Ihrmark K, Boberg J, Trumbore SE, Högberg P, Stenlid J, Finlay RD (2007) Spatial separation of litter decomposition and mycorrhizal nitrogen uptake in a boreal forest. New Phytol 173:611–620
Liu ZG, Zou XM (2002) Exotic earthworms accelerate plant litter decomposition in a Puerto Rican pasture and a wet forest. Ecol Appl 12:1406–1417
Mambelli S, Bird JA, Gleixner G, Dawson TE, Torn MS (2011) Relative contribution of foliar and fine root pine litter to the molecular composition of soil organic matter after in situ degradation. Org Geochem 42:1099–1108
Manzoni S, Jackson RB, Trofymow JA, Porporato A (2008) The global stoichiometry of litter nitrogen mineralization. Science 321:684–686
Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, King JY, Adair EC, Brandt LA, Hart SC, Fasth B (2007) Global-scale similarities in nitrogen release patterns during long-term decomposition. Science 315:361–364
Pregitzer KS, DeForest JL, Burton AJ, Allen MF, Ruess RW, Hendrick RL (2002) Fine root architecture of nine North American trees. Ecol Monog 72:293–309
Prescott CE (2010) Litter decomposition: what controls it and how can we alter it to sequester more carbon in forest soils? Biogeochemistry 101:133–149
Rasse DP, Rumpel C, Dignac MF (2005) Is soil carbon mostly root carbon? Mechanisms for a specific stabilization. Plant Soil 269:341–356
Ryan MG, Melillo JM, Ricca A (1990) A comparison of methods for determining proximate carbon fractions of forest litter. Can J For Res 20:166–171
Seastedt TR, Murray PJ (2008) Root herbivory in grassland ecosystems. In: Johnson SN, Murray PJ (eds) Root feeders: an ecosystem perspective. Cromwell Press, Trowbridge, pp 54–67
Silver WL, Miya RK (2001) Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia 129:407–419
Talbot JM, Treseder KK (2012) Interactions between lignin, cellouse, and nitrogen drive litter chemistry-decay relationships. Ecology (in press) doi: http://dx.doi.org/10.1890/11-0843.1
Taylor BR, Parkinson D, Parsons WFJ (1989) Nitrogen and lignin content as predictors of litter decay-rates-a microcosm test. Ecology 70:97–104
Valenzuela-Estrada LR, Richards JH, Diaz A, Eissenstat DM (2009) Patterns of nocturnal rehydration in root tissues of Vaccinium corymbosum L. under severe drought conditions. J Exp Bot 60:1241–1247
Wang H, Liu S, Mo J (2010) Correlation between leaf litter and fine root decomposition among subtropical tree species. Plant Soil 335:289–298
Wang ZQ, Guo DL, Wang X, Gu J, Mei L (2006) Fine root architecture, morphology, and biomass of different branch orders of two Chinese temperate tree species. Plant Soil 288:151–171
Wells CE, Eissenstat DM (2001) Marked differences in survivorship among apple roots of different diameters. Ecology 82:882–889
Xia M, Guo D, Pregitzer KS (2010) Ephemeral root modules in Fraxinus mandshurica. New Phytol 188:1065–1074
Zhang P, Tian X, He X, Song F, Ren L, Jiang P (2008) Effect of litter quality on its decomposition in broadleaf and coniferous forest. Eur J Soil Biol 44:392–399
Acknowledgements
We thank Mengxue Xia and Zhengxia Chen for assistance in the field and lab. Thanks to Drs. Timothy R. Seastedt and Weixin Cheng for constructive comments on earlier drafts of the manuscript. This research was funded by the Natural Science Foundation of China (NSFC grants 30870418 and 40971030) and by National Basic Research Program of China (No. 2010CB950602).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Johannes Lehmann.
Rights and permissions
About this article
Cite this article
Xiong, Y., Fan, P., Fu, S. et al. Slow decomposition and limited nitrogen release by lower order roots in eight Chinese temperate and subtropical trees. Plant Soil 363, 19–31 (2013). https://doi.org/10.1007/s11104-012-1290-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1290-8