Abstract
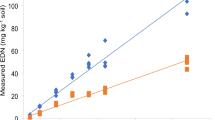
Soil-incorporated plant materials have been associated with reduction in soilborne pathogens and diseases. Mechanisms of the biocidal actions are complex and not well understood. A glasshouse experiment, a non replicated field demonstration, and a field experiment were conducted to determine volatile compounds after incorporation of various plant species and their effect on pest control. Cabbage (Brassica oleracea), canola (Brassica rapa), kale (Brassica oleracea var. acephala), lettuce (Lactuca sativa var. valmaine), two mustard varieties -Caliente (Brassica juncea) and Green wave (Brassica juncea), two radish varieties - Oil seed (Raphanus sativus var. oleiformis) and Cherriette (Raphanus sativus), common rye (Secale cereale), and sorghum Sudan grass (Sorghum bicolor var. sudanese) were used in the glasshouse experiment. Caliente 199 mustard (Brassica hirta) was planted in the field demonstration and white mustard (Sinapis alba) was used in the field experiment. Fresh plant materials were chopped manually in the glasshouse experiment and mechanically in the field studies at the flowering stage before incorporation in natural field soils. In the glasshouse experiment, the equivalent biomass dry weight ranged from a minimum of 573 g m−2 for L. sativa var. valmaine to a maximum of 1851 g m−2 for S. bicolor var. sudanese. The average biomass was 792 g m−2 for B. hirta and 804 g m−2 for S. alba in the two field studies, respectively. The glasshouse experiment used a loamy sand field soil inoculated with a natural fine sandy loam soil that was known to contain high populations of Verticillium dahliae. Soils at both field sites belonged to the sandy loam series, and efforts were made to maintain sufficient soil moisture for plant growth. Although the interest was to determine all volatile compounds in general, only methyl sulfide and dimethyl disulfide were identified and subsequently quantified. Depending on plant species and time of sampling (one to seven days after soil incorporation), 2.7 to 346.4 μg g −1 plant dry weight for methyl sulfide and 0 to 283.2 μg g −1 plant dry weight for dimethyl disulfide were found in the glasshouse experiment. In general, high concentrations of dimethyl disulfide and methyl sulfide appeared to have reduced V. dahliae colony counts in bioassay potato stem saps in the glasshouse experiment. However, the correlation was weak (R 2 = 0.31), but a relatively stronger correlation was obtained (R 2 = 0.58) when excluding B. oleracea and B. rapa from the regression. Dimethyl disulfide and methyl sulfide were nearly non-detectable in the field demonstration, consequently no disease assessment was made. In the field experiment, a production of 5.2 μg g −1 plant dry weight for methyl sulfide and 1.2 μg g −1 dry weight for dimethyl disulfide was found two days after soil incorporation of S. alba. Compared to the untreated control, total Fusarium oxysporum counts in field soil were significantly lower 39 days after S. alba incorporation. However, no significant impact was found on total Pythium counts. Soil population of citrus nematode (Tylenchulus semipenetrans) in the S. alba plots was significantly reduced to similar levels found in the untreated control 112 days after S. alba incorporation. Compared to the untreated control, soil density of non plant parasitic freeliving nematodes was higher 39 days after S. alba incorporation. The study demonstrated quantifiable production of methyl sulfide and dimethyl disulfide gases from a variety of plant species in glasshouse and natural field environments. Some beneficial effects against V. dahliae, F. oxysporum, and T. semipenetrans were observed. Additional studies are needed to further elucidate these complex chemical and biological interactions.



Similar content being viewed by others
References
Arnault I, Vey F, Fleurance C, Nabil H, Auger J (2008) Soil fumigation with Allium sulfur volatiles and Allium by-products. In Proc. Conf. Cultivating the Future Based on Science: 2nd Conference of the International Society of Organic Agriculture Research ISOFAR, Modena, Italy, June 18–20, 2008
Bending GD, Lincoln SD (1999) Characterization of volatile sulphur-containing compounds produced during decomposition of Brassica juncea tissues in soil. Soil Biol Biochem 31:695–703. doi:10.1016/S0038-0717(98) 00163-1
Browne GT, Connell JH, Schneider SM (2006) Almond replant disease and its management with alternative pre-plant soil fumigation treatments and rootstocks. Plant Dis 90:869–876. doi:10.1094/PD-90-0869
Cherr CM, Scholberg JMS, McSorley R (2006) Green manure approaches to crop production: a synthesis. Agron J 98:302–319. doi:10.2134/agronj2005.0035
Cole RA (1976) Isothiocyanates, nitriles and thiocyantes as products of autolysis of glucosinolates in Cruciferae. Phytochem 15:759–762. doi:10.1016/S0031-9422(00) 94437-6
Collins HP, Alva A, Boydston RA, Cochran RL, Hamm PB, McGuire A, Riga E (2006) Soil microbial, fungal, and nematode responses to soil fumigation and cover crops under potato production. Biol Fertil Soils 42:247–257. doi:10.1007/s00374-005-0022-0
Coosemans J (2005) Dimethyl disulphide (DMDS): a potential novel nematicide and soil disinfectant. In Proc. VIth International Symposium on Chemical and non-Chemical Soil and Substrate Disinfestation - SD2004. Vanachter A (Ed), Acta Hort. (ISHS) 698:57–64
Dauphinais N, Bélair G, Fournier Y, Dangi OP (2005) Effect of crop rotation with grain pearl millet on Pratylenchus penetrans and subsequent potato yields in Quebec. Phytoprotection 86:195–199
Davis JR, Huisman OC, Westermann DT, Hafez SL, Everson DO, Sorensen LH, Schneider AT (1996) Effects of green manures on Verticillium wilt of potato. Phytopathology 86:444–453. doi:10.1094/Phyto-86-444
Davis JR, Huisman OC, Westermann DT, Everson DO, Schneider AT, Sorensen LH (2004) Some unique benefits with Sudangrass for improved U.S. #1 yields and size of Russet Burbank potato. Am J Potato Res 81:403–413
Di Primo P, Gamliel A, Austerweil M, Steiner B, Beniches M, Peretz-Alon I, Katan J (2003) Accelerated degradation of metam-sodium and dazomet in soil: characterization and consequences for pathogen control. Crop Prot 22:635–646. doi:10.1016/S0261-2194(03) 00004-8
Flegg FFM, Hooper DJ (1970) Extraction of free-living stages from soil. In: Southey JF (ed) Laboratory methods for work with plant and soil nematodes. Technical Bulletin 2. Ministry of Agriculture, Fisheries and Food, London, pp 5–22
Forney CF, Mattheis JP, Austin RK (1991) Volatile compounds produced by broccoli under anaerobic conditions. J Agric Food Chem 39:2257–2259. doi:10.1021/jf00012a032
Gamliel A, Stapleton JJ (1993) Characterization of antifungal volatile compounds evolved from solarized soil amended with cabbage residues. Phytopathology 83:899–905. doi:10.1094/Phyto-83-899
Gamliel A, Grinstein A, Peretz Y, Klein L, Nachmias A, Tsror L, Livescu L, Katan J (1997) Reduced dosage of methyl bromide for controlling Verticillium wilt of potato in experimental and commercial plots. Plant Dis 81:469–474. doi:10.1094/PDIS.1997.81.5.469
Gerik JS (2005) Evaluation of soil fumigants applied by drip irrigation for Liatris production. Plant Dis 89:883–887. doi:10.1094/PD-89-0883
Gimsing AL, Sorensen JC, Tovgaard L, Jorgensen AMF, Hansen HCB (2006) Degradation kinetics of glucosinolates in soil. Environ Toxicol Chem 25:214–220. doi:10.1897/05-610R.1
Gu YQ, Mo MH, Zhou JP, Zou CS, Zhang KQ (2007) Evaluation and identification of potential organic nematicidal volatiles from soil bacteria. Soil Biol Biochem 39:2567–2575. doi:10.1016/j.soilbio.2007.05.011
Hoyos GP, Zambino PJ, Anderson NA (1991) An assay to quantify vascular colonization of potato by Verticillium dahliae. Am Potato J 68:727–742. doi:10.1007/BF02853804
Kirkegaard JA, Sarwar M (1998) Biofumigation potential of Brassicas. I. Variation in glucosinolate profiles of diverse field-grown Brassicas. Plant Soil 201:71–89. doi:10.1023/A:1004364713152
Komada H (1975) Development of a selective medium for quantitative isolation of Fusarium oxysporum from natural soil. Rev Plant Protec Res 8:114–125
Kubec R, Drhova V, Velisek J (1998) Thermal degradation of s-methylcysteine and its sulfoxide-important flavor precursors of Brassica and Allium vegetables. J Agric Food Chem 46:4334–4340. doi:10.1021/jf980379x
Larkin RP, Griffin TS (2007) Control of soilborne potato diseases using Brassica green manures. Crop Prot 26:1067–1077. doi:10.1016/j.cropro.2006.10.004
Lazarovits G (2001) Management of soil-borne plant pathogens with organic soil amendments: a disease control strategy salvaged from the past. Can J Plant Pathol 23:1–7
Lazzeri L, Baruzzi G, Malaguti L, Antoniacci L (2003) Replacing methyl bromide in annual strawberry production with glucosinolate-containing green manure crops. Pest Manag Sci 59:983–990. doi:10.1002/ps.726
Lewis JA, Papavizas GC (1970) Evolution of sulfur-containing compounds from decomposition of crucifers in soil. Soil Biol Biochem 2:239–246. doi:10.1016/0038-0717(70) 90030-1
Martin FN (1992) The genus Pythium. In: Singleton LL, Mihail JD, Rush CM (eds) Methods for research on soil borne phytopathogenic fungi. American Phytopathological Society, St. Paul, MN, pp 39–49
Matthiessen JN, Kirkegaard JA (2006) Biofumigation and enhanced biodegradation: opportunity and challenge in soilborne pest and disease management. Crit Rev Plant Sci 25:235–265. doi:10.1080/07352680600611543
Mazzola M, Mullinix K (2005) Comparative field efficacy of management strategies containing Brassica napus seed meal or green manure for the control of apple replant disease. Plant Dis 89:1207–1213. doi:10.1094/PD-89-1207
McSorley R (1999) Host suitability of potential cover crops for root-knot nematodes. J Nematol 31:619–623
Mithen RF (2001) Glucosinolates and their degradation products. Adv Bot Res 35:213–262. doi:10.1016/S0065-2296(01) 35008-5
Morra MJ, Kirkegaard JA (2002) Isothiocyanate release from soil-incorporated Brassica tissues. Soil Biol Biochem 34:1683–1690. doi:10.1016/S0038-0717(02) 00153-0
Raaijmakers JM, Paulitz TC, Steinberg C, Alabouvette C, Moënne-Loccoz Y (2009) The rhizosphere: a playground and battlefield for soilborne pathogens and beneficial microorganisms. Plant Soil
Rosskopf EN, Church G, Holzinger J, Yandoc-Ables C, Noling J (2006) Efficacy of dimethyl disulfide (DMDS) for control of nematodes and fungal plant pathogens. Phytopathology 96:S100
Sang JP, Minchinton IR, Johnstone PK, Truscott RJ (1984) Glucosinolate profiles in the seed, root and leaf tissue of cabbage, mustard, rapeseed, radish and swede. Can J Plant Sci 64:77–93
Sarwar M, Kirkegaard JA, Wong PTW, Desmarchelier JM (1998) Biofumigation potential of BrassicasIII. In vitro toxicity of isothiocyanates to soilborne fungal pathogens. Plant Soil 201:103–112. doi:10.1023/A:1004381129991
Schneider SM, Ajwa HA, Trout TJ (2006) Chemical alternatives to methyl bromide for vineyard replant conditions. Am J Enol Vitic 57:183–193
Smolinska U, Horbowicz M (1999) Fungicidal activity of volatiles from selected cruciferous plants against resting propagules of soil-borne fungal pathogens. Phytopathology 147:119–124
Snapp SS, Date KU, Kirk W, Neil KO, Kremen A, Bird G (2007) Root, shoot tissues of Brassica juncea and Cereal secale promote potato health. Plant Soil 294:55–72. doi:10.1007/s11104-007-9228-2
Subbarao KV, Kabir Z, Martin FN, Koike ST (2007) Management of soilborne diseases in strawberry using vegetable rotations. Plant Dis 91:964–972. doi:10.1094/PDIS-91-8-0964
Walker JT (1996) Crambe and rapeseed meal as soil amendments: nematicidal potential and phytotoxic effects. Crop Prot 15:433–437. doi:10.1016/0261-2194(96) 00001-4
Warton B, Matthiessen JN, Shackleton MA (2001) Glucosinolate content and isothiocyanate evolution-two measures of the biofumigation potential of plants. J Agric Food Chem 49:5244–5250. doi:10.1021/jf010545s
Westphal A, Browne GT, Schneider S (2002) Evidence for biological nature of the grape replant problem in California. Plant Soil 242:197–203. doi:10.1023/A:1016297603427
Wiggins BE, Kinkel LL (2005) Green manures and crop sequences influence potato diseases amd pathogen inhibitory activity of indigenous streptomycetes. Phytopathology 95:178–185. doi:10.1094/PHYTO-95-0178
Wilhelm S, Paulus AO (1980) How soil fumigation benefits the California strawberry industries. Plant Dis 64:264–270
Wong PK, Wang YH (1997) Determination of the Henry’s law constant for dimethyl sulfide in seawater. Chemosphere 35:535–544. doi:10.1016/S0045-6535(97) 00118-5
Yulianti T, Sivasithamparam K, Turner DW (2007) Saprophytic and pathogenic behaviour of R. solani AG2-1 (ZG-5) in a soil amended with Diplotaxis tenuifolia or Brassica nigra manures and incubated at different temperatures and soil water content. Plant Soil 294:277–289. doi:10.1007/s11104-007-9254-0
Zasada IA, Ferris H (2004) Nematode suppression with brassicaceous amendments: application based upon glucosinolate profiles. Soil Biol Biochem 36:1017–1024. doi:10.1016/j.soilbio.2003.12.014
Zhang Y, Spokas K, Wang D (2005) Degradation of methyl isothiocyanate and chloropicrin in forest nursery soils. J Environ Qual 34:1566–1572. doi:10.2134/jeq2004.0374
Acknowledgments
The authors would like to thank Matt McNearney, Kun Xiao, Josh Zimmerman, and Chuck Hyatt for general help in the Minnesota experiments; Jim Gartung, Tom Pflaum, Nancy Goodell, Curtis Koga, and Patricia Mungur for the California experiment. We are grateful to Dale Gies of Hi-Performance Seeds, Inc. (Moses Lake, WA) for donating the mustard seeds and John Petron (Long Prairie, MN) for letting us use his field and for planting and incorporating the mustard crop in the soil. Constructive comments on the manuscript from Susanne Klose, Mark Mazzola, and Erin Rosskopf are greatly appreciated. We also want to acknowledge grants from the University of Minnesota IREE program and USDA-ARS Pacific Area-wide Methyl Bromide Alternatives program.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Peter A.H. Bakker.
Rights and permissions
About this article
Cite this article
Wang, D., Rosen, C., Kinkel, L. et al. Production of methyl sulfide and dimethyl disulfide from soil-incorporated plant materials and implications for controlling soilborne pathogens. Plant Soil 324, 185–197 (2009). https://doi.org/10.1007/s11104-009-9943-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-009-9943-y