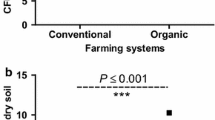
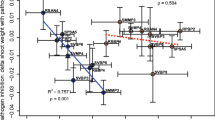
Abstract
Amendment of orchard soil with low-glucosinolate Brassica napus (rape) seed meal (RSM) suppresses infection of apple roots by Rhizoctonia solani but increases incidence of Pythium spp. infection. Following incorporation of Brassica sp. seed meals, soils were monitored for changes in populations of selected saprophytic and plant pathogenic microorganisms. When conducted in pasteurized soil, which possessed high numbers of Bacillus spp. and lower than detectable numbers of Streptomyces spp., RSM amendment did not provide control of R. solani. Populations of streptomycetes in RSM-amended soil increased to stable levels >20-fold higher than in non-amended soil. Disease suppressiveness was restored to pasteurized RSM-amended soil by adding any of several Streptomyces strains. Maximal rates of nitrification in orchard soil, determined by nitric oxide emission, were observed within two weeks following RSM amendment and inhibition of nitrification via application of nitrapyrin abolished the capacity of RSM to suppress R. solani infection of apple roots when seedlings were planted one day after soil amendment. Apple seedling mortality and Pythium spp. root infection were highest for seedlings planted immediately following incorporation of B. napus cv. Athena RSM, particularly when meal was added in a flake rather than powder form. Lower infection frequencies were observed for seedlings planted four weeks after RSM incorporation, even for soil in which densities of culturable Pythium spp. had not declined. Our results demonstrate that suppression of Rhizoctonia root rot in response to RSM amendment requires the activity of the resident soil microbiota and that initial disease control is associated with the generation of nitric oxide through the process of nitrification.
Similar content being viewed by others
References
Borek V, Morra MJ (2005) Ionic Thiocyanate (SCN-) production from 4- hydroxybenzyl glucosinolate contained in Sinapis alba seed meal. J Agric Food Chem. 53:8650–8654
Brabban AD, Edwards C (1996) Characterization of growth and product formation by a thermophilic␣streptomycete grown in a particulate rapemeal-derived liquid medium. J Appl Bacteriol 80:651–658
Challis GL, Hopwood DA (2003) Synergy and contingency as driving forces for the evolution of multiple secondary metabolite production by Streptomyces species. Proc Nat Acad Sci USA 100:14555–14561
Cohen MF, Mazzola M (2005) Suppression of Rhizoctonia root rot by Streptomyces in Brassica napus seed meal-amended soil. Phytopathology 95:S20
Cohen MF, Mazzola M, Yamasaki H (2006) Nitric oxide research in agriculture: bridging the plant and bacterial realms. In: Rai AK, Takabe T (eds) Genetic engineering of stress tolerant plants. Springer, Dordrecht, pp 71–90
Cohen MF, Yamasaki H, Mazzola M (2005) Modification of microbial community structure, nitric oxide production and incidence of Rhizoctonia root rot in response to Brassica napus seed meal soil amendment. Soil Biol Biochem 37:1215–1227
Girlanda M, Perotto S, Moenne-Loccoz Y, Bergero R, Lazzari A, Defago G, Bonfante P, Luppi AM (2001) Impact of biocontrol Pseudomonas fluorescens CHA0 and a genetically modified derivative on the diversity of culturable fungi in the cucumber rhizosphere. Appl Environ Microbiol 67:1851–1864
Hendrix FF, Campbell WA (1973) Pythiums as plant pathogens. Annu Rev Phytopathol 11:77–98
Huber DM, Watson RD, Steiner GW (1965) Crop residues, nitrogen, and plant disease. Soil Sci 100:302–308
Igarashi Y, Iida T, Sasaki T, Saito N, Yoshida R, Furumai T (2002) Isolation of actinomycetes from live plants and evaluation of antiphytopathogenic activity of their metabolites. Actinomycetologica 16:9–13
Jeffers SN, Aldwinckle HS, Burr TJ, Arneson PA (1982) Phytophthora and Pythium species associated with crown rot in New York apple orchards. Phytopathology 72:533–538
Lahdenpera M-L, Simon E, Uoti J (1991) Mycostop-a novel biofungicide based on streptomyces bacteria. In: Beemster ABR, Bollen GJ, Gerlagh M, Ruissen MA, Schippers B, Temple A (eds) Biotic interactions and soil-borne diseases. Elsevier, Amsterdam, pp 253–263
Manici LM, Lazzeri L, Palmieri SJ (1997) In vitro fungitoxic activity of some glucosinolates and their enzyme-derived products toward plant pathogenic fungi. J Agric Food Chem 45:2768–2773
Mazzola M (1997) Identification and pathogenicity of Rhizoctonia spp. isolated from apple roots and orchard soil. Phytopathology 87:582–587
Mazzola M (1998) Elucidation of the microbial complex having a causal role in the development of apple replant disease in Washington. Phytopathology 88:930–938
Mazzola M (1999) Transformation of soil microbial community structure and Rhizoctonia-suppressive potential in response to apple roots. Phytopathology 89:920–927
Mazzola M, Andrews PK, Reganold JP, Lévesque CA (2002) Frequency, virulence, and metalxyl sensitivity of Pythium spp. isolated from apple roots under conventional and organic production systems. Plant Dis 86:669–675
Mazzola M, Granatstein DM, Elfving DC, Mullinix K (2001) Suppression of specific apple root pathogens by Brassica napus seed meal amendment regardless of glucosinolate content. Phytopathology 91:673–679
Mazzola M, Mullinix K (2005) Comparative field efficacy of measures containing Brassica napus seed meal or green manure for the management of apple replant disease. Plant Dis 89:1207–1213
Mazzola M, Wong OT, Cook RJ (1996) Virulence of Rhizoctonia oryzae and R. solani AG-8 on wheat and detection of R. oryzae in plant tissue by PCR. Phytopathology 86:354–360
Neill SJ, Desikan R, Hancock JT (2003) Nitric oxide signalling in plants. New Phytol 159:11–35
Rothrock CS, Gottlieb D (1984) Role of antibiosis of Streptomyces hygroscopicus var. geldanus to Rhizoctonia solani in soil. Can J Microbiol 30:1440–1447
Shimizu M, Furumai T, Igarashi Y, Onaka H, Nishimura T, Yoshida R, Kunoh H (2001) Association of induced disease resistance of rhododendron seedlings with inoculation of Streptomyces sp. R-5 and treatment with actinomycin D and amphotericin B to the tissue-culture medium. J Antibio 54:501–505
Taylor RJ, Salas B, Secor GA, Rivera V, Gudmestad NC (2002) Sensitivity of North American isolates of Phytophthora erythroseptica and Pythium ultimum to mefenoxam (metalaxyl). Plant Dis 86:797–802
Tokala RK, Strap JL, Jung CM, Crawford DL, Salove MH, Deobald LA, Bailey JF, Morra MJ (2002) Novel plant-microbe rhizosphere interaction involving Streptomyces lydicus WYEC108 and the pea plant (Pisum sativum). Appl Environ Microbiol 68:2161–2171
van Loon LC, Bakker PAHM, Pieterse CMJ (1998) Systemic resistance induced by rhizosphere bacteria. Annu Rev Phytopathol 36:453–483
Weller DM, Raaijmakers JM, Gardner BBM, Thomashow LS (2002) Microbial populations responsible for specific soil suppressiveness to plant pathogens. Annu Rev Phytopathol 40:309–348
Williams OB, Lamanna C, Knaysi G, Wynne ES, Schmidt CF, Curran HR, Levine M, Sugiyama H, Reynolds H, Lichtenstein H, Phillips CR (1952) Symposium on the biology of bacterial spores. Bacteriol Rev 16:89–143
Acknowledgements
We are grateful for the technical assistance of Sheila Ivanov and Dr. Mami Kainuma. This work was funded in part through grants to M. Mazzola from the USDA CSREES Organic and Integrated Grants Program and the Washington Tree Fruit Research Commission.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cohen, M.F., Mazzola, M. Resident bacteria, nitric oxide emission and particle size modulate the effect of Brassica napus seed meal on disease incited by Rhizoctonia solani and Pythium spp. Plant Soil 286, 75–86 (2006). https://doi.org/10.1007/s11104-006-9027-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-006-9027-1