Abstract
Key message
Here we first found that GsERF71, an ERF factor from wild soybean could increase plant alkaline stress tolerance by up-regulating H+-ATPase and by modifing the accumulation of Auxin.
Abstract
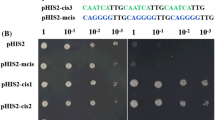
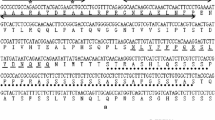
Alkaline soils are widely distributed all over the world and greatly limit plant growth and development. In our previous transcriptome analyses, we have identified several ERF (ethylene-responsive factor) genes that responded strongly to bicarbonate stress in the roots of wild soybean G07256 (Glycine soja). In this study, we cloned and functionally characterized one of the genes, GsERF71. When expressed in epidermal cells of onion, GsERF71 localized to the nucleus. It can activate the reporters in yeast cells, and the C-terminus of 170 amino acids is essential for its transactivation activity. Yeast one-hybrid and EMSA assays indicated that GsERF71 specifically binds to the cis-acting elements of the GCC-box, suggesting that GsERF71 may participate in the regulation of transcription of the relevant biotic and abiotic stress-related genes. Furthermore, transgenic Arabidopsis plants overexpressing GsERF71 showed significantly higher tolerance to bicarbonate stress generated by NaHCO3 or KHCO3 than the wild type (WT) plants, i.e., the transgenic plants had greener leaves, longer roots, higher total chlorophyll contents and lower MDA contents. qRT-PCR and rhizosphere acidification assays indicated that the expression level and activity of H+-ATPase (AHA2) were enhanced in the transgenic plants under alkaline stress. Further analysis indicated that the expression of auxin biosynthetic genes and IAA contents were altered to a lower extent in the roots of transgenic plants than WT plants under alkaline stress in a short-term. Together, our data suggest that GsERF71 enhances the tolerance to alkaline stress by up-regulating the expression levels of H+-ATPase and by modifying auxin accumulation in transgenic plants.










Similar content being viewed by others
Abbreviations
- WT:
-
Wild type
- OX:
-
Overexpression
- TF:
-
Transcription factor
References
Brand AH, Breeden L, Abraham J, Sternglanz R, Nasmyth K (1985) Characterization of a “silencer” in yeast: a DNA sequence with properties opposite to those of a transcriptional enhancer. Cell 41:41–48
Cao Y, Song F, Goodman RM, Zheng Z (2006) Molecular characterization of four rice genes encoding ethylene-responsive transcriptional factors and their expressions in response to biotic and abiotic stress. J Plant Physiol 163:1167–1178
Carter Jr TE, Nelson RL, Sneller C, Cui Z. (2004) Genetic diversity in soybean. In: Boerma HR, Specht JE, ed. Soybean: improvement, production, and uses, 3rd edn. Madison: American Society for Agronomy. pp. 303–416
Chaney RL, Coulombe BA, Bell PF, Angle JS (1992) Detailed method to screen dicot cultivars for resistance to Fe‐chlorosis using FeDTPA and bicarbonate in nutrient solutions. J Plant Nutr 15:2063–2083
Chen T, Yang Q, Gruber M, Kang J, Sun Y, Ding W, Zhang T, Zhang X (2012) Expression of an alfalfa (Medicago sativa L.) ethylene response factor gene MsERF8 in tobacco plants enhances resistance to salinity. Mol Biol Rep 39:6067–6075
Cheng Y, Dai X, Zhao Y (2007) Auxin synthesized by the YUCCA flavin monooxygenases is essential for embryogenesis and leaf formation in Arabidopsis. Plant Cell 19:2430–2439
Cheng MC, Liao PM, Kuo WW, Lin TP (2013) The Arabidopsis ETHYLENE RESPONSE FACTOR1 regulates abiotic stress-responsive gene expression by binding to different cis-acting elements in response to different stress signals. Plant Physiol 162:1566–1582
Deokar AA, Kondawar V, Kohli D, Aslam M, Jain PK, Karuppayil SM, Varshney RK, Srinivasan R (2015) The CarERF genes in chickpea (Cicer arietinum L.) and the identification of CarERF116 as abiotic stress responsive transcription factor. Funct Integr Genomic 15:27–46
DuanMu H, Wang Y, Bai X, Cheng S, Deyholos MK, Wong GK, Li D, Zhu D, Li R, Yu Y, Cao L, Chen C, Zhu Y (2015) Wild soybean roots depend on specific transcription factors and oxidation reduction related genesin response to alkaline stress. Funct Integr Genomic 15:651–660
FRELIN C, VIGNE P, LADOUX A, LAZDUNSKI M (1988) The regulation of the intracellular pH in cells from vertebrates. Eur J Biochem 174:3–14
Fu X, Harberd NP (2003) Auxin promotes Arabidopsis root growth by modulating gibberellin response. Nature 421:740–743
Fuglsang AT, Guo Y, Cuin TA, Qiu Q, Song C, Kristiansen KA, Bych K, Schulz A, Shabala S, Schumaker KS, Palmgren MG, Zhu JK (2007) Arabidopsis protein kinase PKS5 inhibits the plasma membrane H+-ATPase by preventing interaction with 14-3-3 protein. Plant Cell 19:1617–1634
Fushimi T, Umeda M, Shimazaki T, Kato A, Toriyama K, Uchimiya H (1994) Nucleotide sequence of a rice cDNA similar to a maize NADP-dependent malic enzyme. Plant Mol Biol 24:965–967
Gasch P, Fundinger M, Muller JT, Lee T, Bailey-Serres J, Mustroph A (2016) Redundant ERF-VII transcription factors bind to an evolutionarily conserved cis-motif to regulate hypoxia-responsive gene expression in Arabidopsis. Plant Cell 28:160–180
Ge Y, Li Y, Zhu YM, Bai X, Lv DK, Guo D, Ji W, Cai H (2010) Global transcriptome profiling of wild soybean (Glycine soja) roots under NaHCO3 treatment. BMC Plant Biol 10:153
Grieneisen VA, Xu J, Maree AFM, Hogeweg P, Scheres B (2007) Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature 449:1008–1013
Guo J, Liu Y, Wang Y, Chen J, Li Y, Huang H, Qiu L, Wang Y (2012) Population structure of the wild soybean (Glycine soja) in China: implications from microsatellite analyses. Ann Bot 110:777–785
Hager A (2003) Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects. J Plant Res 116:483–505
He S, Wang Y, Volis S, Li D, Yi T (2012) Genetic diversity and population structure: implications for conservation of wild soybean (Glycine soja Sieb. et Zucc) based on nuclear and chloroplast microsatellite variation. INT J Mol Sci 13:12608–12628
Hinz M, Wilson IW, Yang J, Buerstenbinder K, Llewellyn D, Dennis ES, Sauter M, Dolferus R (2010) Arabidopsis RAP2.2: an ethylene response transcription factor that is important for hypoxia survival. Plant Physiol 153:757–772
Hu N, Tang N, Yan F, Bouzayen M, Li ZG (2014) Effect of LeERF1 and LeERF2 overexpression in the response to salinity of young tomato (Solanum lycopersicum cv. micro-tom) seedlings. Acta Physiol Plant 36:1703–1712
Jin H, Plaha P, Park JY, Hong CP, Lee IS, Yang ZH, Jiang GB, Kwak SS, Liu SK, Lee JS, Kim YA, Lim YP (2006) Comparative EST profiles of leaf and root of Leymus chinensis, a xerophilous grass adapted to high pH sodic soil. Plant Sci 170:1081–1086
Kawanabe S, Zhu TC (1991) Degeneration and conservation of Aneurolepisium chinense grassland in Northern China. J Japam Grassland Sci 37:91–99
Kleine-Vehn J, Langowski L, Wisniewska J, Dhonukshe P, Brewer PB, Friml J (2008) Cellular and molecular requirements for polar PIN targeting and transcytosis in plants. Mol Plant 1:1056–1066
Kobayashi S, Satone H, Tan E, Kurokochi H, Asakawa S, Liu S, Takano T (2015) Transcriptional responses of a bicarbonate-tolerant monocot, Puccinellia tenuiflora, and a related bicarbonate-sensitive species, Poa annua, to NaHCO3 stress. Int J Mol Sci 16:496–509
Korasick DA, Enders TA, Strader LC (2013) Auxin biosynthesis and storage forms. J Exp Bot 64:2541–2555
Lee JH, Hong JP, Oh SK, Lee S, Choi D, Kim WT (2004) The ethylene-responsive factor like protein 1 (CaERFLP1) of hot pepper (Capsicum annuum L.) interacts in vitro with both GCC and DRE/CRT sequences with different binding affinities: possible biological roles of CaERFLP1 in response to pathogen infection and high salinity conditions in transgenic tobacco plants. Plant Mol Biol 55:61–81
Lee SY, Hwang EY, Seok HY, Tarte VN, Jeong MS, Jang SB, Moon YH (2015) Arabidopsis AtERF71/HRE2 functions as transcriptional activator via cis-acting GCC box or DRE/CRT element and is involved in root development through regulation of root cell expansion. Plant Cell Rep 34:223–231
Lee DK, Jung H, Jang G, Jeong JS, Kim YS, Ha SH, Choi YD, Kim JK (2016) Overexpression of the OsERF71 transcription factor alters rice root structure and drought resistance. Plant Physiol 172:575–588
Li J, Xu HH, Liu WC, Zhang XW, Lu YT (2015) Ethylene inhibits root elongation during alkaline stress through AUXIN1 and associated changes in auxin accumulation. Plant Physiol 168:1777–1791
Licausi F, Kosmacz M, Weits DA, Giuntoli B, Giorgi FM, Voesenek LA, Perata P, van Dongen JT (2011) Oxygen sensing in plants is mediated by an N-end rule pathway for protein destabilization. Nature 479:419–422
Liu J, Guo Y (2011) The alkaline tolerance in Arabidopsis requires stabilizing microfilament partially through inactivation of PKS5 kinase. J Genet Genom 38:307–313
Liu ZB, Hagen G, Guilfoyle TJ (1997) A G-box-binding protein from soybean binds to the E1 auxin-response element in the soybean GH3 promoter and contains a proline-rich repression domain. Plant Physiol 115:397–407
Liu S, Cheng Y, Zhang X, Guan Q, Nishiuchi S, Hase K, Takano T (2007) Expression of an NADP-malic enzyme gene in rice (Oryza sativa. L) is induced by environmental stresses; over-expression of the gene in Arabidopsis confers salt and osmotic stress tolerance. Plant Mol Biol 64:49–58
Liu A, Yu Y, Duan X, Sun X, Duanmu H, Zhu Y (2015a) GsSKP21, a Glycine soja S-phase kinase-associated protein, mediates the regulation of plant alkaline tolerance and ABA sensitivity. Plant Mol Biol 87:111–124
Liu W, Li RJ, Han TT, Cai W, Fu ZW, Lu YT (2015b) Salt stress reduces root meristem size by nitric oxide-mediated modulation of auxin accumulation and signaling in Arabidopsis. Plant Physiol 168:343–356
Ludwig-Muller J (2011) Auxin conjugates: their role for plant development and in the evolution of land plants. J Exp Bot 62:1757–1773
Ma Y, Zhang L, Zhang J et al (2014) Expressing a citrus ortholog of arabidopsis ERF1 enhanced cold-tolerance in tobacco. Sci Hortic 174:65–76
Makhloufi E, Yousfi FE, Marande W, Mila I, Hanana M, Berges H, Mzid R, Bouzayen M (2014) Isolation and molecular characterization of ERF1, an ethylene response factor gene from durum wheat (Triticum turgidum L. subsp. durum), potentially involved in salt-stress responses. J Exp Bot 65:6359–6371
Mao JL, Miao ZQ, Wang Z, Yu LH, Cai XT, Xiang CB (2016) Arabidopsis ERF1 mediates cross-talk between ethylene and auxin biosynthesis during primary root elongation by regulating ASA1 expression. PLoS Genet 12(1):e1005760
Marrè E, Ballarin-Denti A (1985) The proton pumps of the plasmalemma and the tonoplast of higher plants. J Bioenerg Biomembr 17:1–21
Mazarei M, Elling AA, Maier TR, Puthoff DP, Baum TJ (2007) GmEREBP1 is a transcription factor activating defense genes in soybean and Arabidopsis. Mol Plant Microbe Interact 20:107–119
Mishra S, Phukan UJ, Tripathi V, Singh DK, Luqman S, Shukla RK (2015) PsAP2 an AP2/ERF family transcription factor from Papaver somniferum enhances abiotic and biotic stress tolerance in transgenic tobacco. Plant Mol Biol 89:173–186
Mizoi J, Shinozaki K, Yamaguchi-Shinozaki K (2012) AP2/ERF family transcription factors in plant abiotic stress responses. BBA-Gene Regul Mech 1819:86–96
Moloney MM, Elliott MC, Cleland RE (1981) Acid growth effects in maize roots: Evidence for a link between auxin-economy and proton extrusion in the control of root growth. Planta 152:285–291
Muller M, Munne-Bosch S (2015) Ethylene response factors: a key regulatory hub in hormone and stress signaling. Plant Physiol 169:32–41
Nakano T, Suzuki K, Fujimura T, Shinshi H (2006) Genome-wide analysis of the ERF gene family in Arabidopsis and rice. Plant Physiol 140:411–432
Nasmyth K, Shore D (1987) Transcriptional regulation in the yeast life cycle. Science 237:1162–1170
Onate-Sanchez L, Anderson JP, Young J, Singh KB (2007) AtERF14, a member of the ERF family of transcription factors, plays a nonredundant role in plant defense. Plant Physiol 143:400–409
Papdi C, Perez-Salamo I, Joseph MP, Giuntoli B, Bogre L, Koncz C, Szabados L (2015) The low oxygen, oxidative and osmotic stress responses synergistically act through the ethylene response factor VII genes RAP2.12, RAP2.2 and RAP2.3. Plant J 82:772–784
Park JM, Park CJ, Lee SB, Ham BK, Shin R, Paek KH (2001) Overexpression of the tobacco Tsi1 gene encoding an EREBP/AP2-type transcription factor enhances resistance against pathogen attack and osmotic stress in tobacco. Plant Cell 13:1035–1046
Park JE, Park JY, Kim YS, Staswick PE, Jeon J, Yun J, Kim SY, Kim J, Lee YH, Park CM (2007) GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J Biol Chem 282:10036–10046
Park HY, Seok HY, Woo DH, Lee SY, Tarte VN, Lee EH, Lee CH, Moon YH (2011) AtERF71/HRE2 transcription factor mediates osmotic stress response as well as hypoxia response in Arabidopsis. Biochem Bioph Res Co 414:135–141
Qi X, Li MW, Xie M, Liu X, Ni M, Shao G, Song C, Kay-Yuen Yim A, Tao Y, Wong FL, Isobe S, Wong CF, Wong KS, Xu C, Li C, Wang Y, Guan R, Sun F, Fan G, Xiao Z, Zhou F, Phang TH, Liu X, Tong SW, Chan TF, Yiu SM, Tabata S, Wang J, Xu X, Lam HM (2014) Identification of a novel salt tolerance gene in wild soybean by whole-genome sequencing. Nat Commun 5:4340
Rayle DL, Cleland R (1970) Enhancement of wall loosening and elongation by acid solutions. Plant Physiol 46:250–253
Rayle DL, Cleland RE (1980) evidence that auxin-induced growth of soybean hypocotyls involves proton excretion. Plant Physiol 66:433–437
Rong W, Qi L, Wang A, Ye X, Du L, Liang H, Xin Z, Zhang Z (2014) The ERF transcription factor TaERF3 promotes tolerance to salt and drought stresses in wheat. Plant Biotechnol J 12:468–479
Sakuma Y, Liu Q, Dubouzet JG, Abe H, Shinozaki K, Yamaguchi-Shinozaki K (2002) DNA-binding specificity of the ERF/AP2 domain of Arabidopsis DREBs, transcription factors involved in dehydration- and cold-inducible gene expression. Biochem Bioph Res Co 290:998–1009
Sharoni AM, Nuruzzaman M, Satoh K, Shimizu T, Kondoh H, Sasaya T, Choi IR, Omura T, Kikuchi S (2011) Gene structures, classification and expression models of the AP2/EREBP transcription factor family in rice. Plant Cell Physiol 52:344–360
Shoji T, Mishima M, Hashimoto T (2013) Divergent DNA-binding specificities of a group of ethylene response factor transcription factors involved in plant defense. Plant Physiol 162:977–990
Solano R, Stepanova A, Chao Q, Ecker JR (1998) Nuclear events in ethylene signaling: a transcriptional cascade mediated by ethylene-insensitive3 and ethylene-response-factor1. Gene Dev 12:3703–3714
Song X, Li Y, Hou X (2013) Genome-wide analysis of the AP2/ERF transcription factor superfamily in Chinese cabbage (Brassica rapa ssp. pekinensis). BMC Genom 14:573
Stockinger EJ, Gilmour SJ, Thomashow MF (1997) Arabidopsis thaliana CBF1 encodes an AP2 domain-containing transcriptional activator that binds to the C-repeat/DRE, a cis-acting DNA regulatory element that stimulates transcription in response to low temperature and water deficit. Pnatl Acad Sci USA 94:1035–1040
Sun ZM, Zhou ML, Xiao XG, Tang YX, Wu YM (2014) Genome-wide analysis of AP2/ERF family genes from Lotus corniculatus shows LcERF054 enhances salt tolerance. Funct Integr Genom 14:453–466
Takahashi K, Hayashi K, Kinoshita T (2012) Auxin activates the plasma membrane H+-ATPase by phosphorylation during hypocotyl elongation in Arabidopsis. Plant Physiol 159:632–641
Torimitsu K, Yazaki Y, Nagasuka K, Ohta E, Sakata M (1984) Effect of external pH on the cytoplasmic and vacuolar pHs in mung bean root-tip cells: A 31P nuclear magnetic resonance study. Plant Cell Physiol 25:1403–1409
Tournier B, Sanchez-Ballesta MT, Jones B, Pesquet E, Regad F, Latche A, Pech JC, Bouzayen M (2003) New members of the tomato ERF family show specific expression pattern and diverse DNA-binding capacity to the GCC box element. FEBS Lett 550:149–154
Trujillo LE, Sotolongo M, Menendez C, Ochogavia ME, Coll Y, Hernandez I, Borras-Hidalgo O, Thomma BP, Vera P, Hernandez L (2008) SodERF3, a novel sugarcane ethylene responsive factor (ERF), enhances salt and drought tolerance when overexpressed in tobacco plants. Plant Cell Physiol 49:512–525
Wang YC, Ma H, Liu GF, Xu CX, Zhang DW, Ban QY (2008) Analysis of Gene Expression Profile of Limonium bicolor under NaHCO3 Stress Using cDNA Microarray. Plant Mol Biol Rep 26:241–254
Wang L, Qin L, Liu W, Zhang D, Wang Y (2014) A novel ethylene-responsive factor from Tamarix hispida, ThERF1, is a GCC-box- and DRE-motif binding protein that negatively modulates abiotic stress tolerance in Arabidopsis. Physiol Plant 152:84–97
Wang X, Liu S, Tian H, Wang S, Chen JG (2015) The small ethylene response factor ERF96 is involved in the regulation of the abscisic acid response in Arabidopsis. Front Plant Sci 6:1064. doi:10.3389/fpls.2015.01064
Weber H, Chételat A, Reymond P, Farmer EE (2004) Selective and powerful stress gene expression in Arabidopsis in response to malondialdehyde. Plant J 37:877–888
Xu ZS, Xia LQ, Chen M, Cheng XG, Zhang RY, Li LC, Zhao YX, Lu Y, Ni ZY, Liu L, Qiu ZG, Ma YZ (2007) Isolation and molecular characterization of the Triticum aestivum L. ethylene-responsive factor 1 (TaERF1) that increases multiple stress tolerance. Plant Mol Biol 65:719–732
Xu W, Jia L, Baluska F, Ding G, Shi W, Ye N, Zhang J (2012) PIN2 is required for the adaptation of Arabidopsis roots to alkaline stress by modulating proton secretion. J Exp Bot 63:6105–6114
Yamaguchi-Shinozaki K, Shinozaki K (1994) A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell 6:251–264
Yang Z, Tian LN, Latoszek-Green M, Brown D, Wu KQ (2005) Arabidopsis ERF4 is a transcriptional repressor capable of modulating ethylene and abscisic acid responses. Plant Mol Biol 58:585–596
Yang CW, Chong JN, Li CY, Kim CM, Shi DC, Wang DL (2007) Osmotic adjustment and ion balance traits of an alkali resistant halophyte Kochia sieversiana during adaptation to salt and alkali conditions. Plant Soil 294:263–276
Yang CW, Wang P, Li CY, Shi DC, Wang DL (2008) Comparison of effects of salt and alkali stresses on the growth and photosynthesis of wheat. Photosynthetica 46:107–114
Yang CW, Xu HH, Wang LL, Liu J, Shi DC, Wang DL (2009) Comparative effects of salt-stress and alkali-stress on the growth, photosynthesis, solute accumulation, and ion balance of barley plants. Photosynthetica 47:79–86
Yang Y, Qin Y, Xie C, Zhao F, Zhao J, Liu D, Chen S, Fuglsang AT, Palmgren MG, Schumaker KS, Deng XW, Guo Y (2010) The Arabidopsis chaperone J3 regulates the plasma membrane H+-ATPase through interaction with the PKS5 kinase. Plant Cell 22:1313–1332
Yang CY, Hsu FC, Li JP et al (2011) The AP2/ERF transcription factor AtERF73/HRE1 modulates ethylene responses during hypoxia in Arabidopsis. Plant Physiol 156:202–212
Yang H, Yu C, Yan J, Wang X, Chen F, Zhao Y, Wei W (2014) Overexpression of the Jatropha curcas JcERF1 gene coding an AP2/ERF-type transcription factor increases tolerance to salt in transgenic tobacco. BioChemistry 79:1226–1236
Yi Y, Guerinot ML (1996) Genetic evidence that induction of root Fe(III) chelate reductase activity is necessary for iron uptake under iron deficiency. Plant J 10:835–844
Yi SY, Kim JH, Joung YH, Lee S, Kim WT, Yu SH, Choi D (2004) The pepper transcription factor CaPF1 confers pathogen and freezing tolerance in Arabidopsis. Plant Physiol 136:2862–2874
Youm JW, Jeon JH, Choi D, Yi SY, Joung H, Kim HS (2008) Ectopic expression of pepper CaPF1 in potato enhances multiple stresses tolerance and delays initiation of in vitro tuberization. Planta 228:701–708
Yu Y, Liu A, Duan X, Wang S, Sun X, Duanmu H, Zhu D, Chen C, Cao L, Xiao J, Li Q, Nisa ZU, Zhu Y, Ding X (2016) GsERF6, an ethylene-responsive factor from Glycine soja, mediates the regulation of plant bicarbonate tolerance in Arabidopsis. Planta 244:681–698
Zhang Z, Huang R (2010) Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Molecular Biol 73:241–249
Zhang G, Chen M, Chen X, Xu Z, Guan S, Li LC, Li A, Guo J, Mao L, Ma Y (2008) Phylogeny, gene structures, and expression patterns of the ERF gene family in soybean (Glycine max L.). J Exp Bot 59:4095–4107
Zhang GY, Chen M, Li LC, Xu ZS, Chen XP, Guo JM, Ma YZ (2009) Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought, and diseases in transgenic tobacco. J Exp Bot 60:3781–3796
Zhang GY, Chen M, Chen XP, Xu ZS, Li LC, Guo JM, Ma YZ (2010) Isolation and characterization of a novel EAR-motif-containing gene GmERF4 from soybean (Glycine max L.). Mol Biol Rep 37:809–818. doi:10.1007/s11033-009-9616-1
Zhang Z, Wang J, Zhang R, Huang R (2012) The ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis. Plant J 71:273–287
Zhao Y, Wei T, Yin KQ, Chen Z, Gu H, Qu LJ, Qin G (2012) Arabidopsis RAP2.2 plays an important role in plant resistance to Botrytis cinerea and ethylene responses. New Phytol 195:450–460
Zhu D, Bai X, Chen C, Chen Q, Cai H, Li Y, Ji W, Zhai H, Lv D, Luo X (2011) GsTIFY10, a novel positive regulator of plant tolerance to bicarbonate stress and a repressor of jasmonate signaling. Plant Mol Biol 77:285–297
Zhu D, Cai H, Luo X, Bai X, Deyholos MK, Chen Q, Chen C, Ji W, Zhu Y (2012) Over-expression of a novel JAZ family gene from Glycine soja, increases salt and alkali stress tolerance. Biochem Bioph Res Co 426:273–279
Zhuang J, Cai B, Peng RH, Zhu B, Jin XF, Xue Y, Gao F, Fu XY, Tian YS, Zhao W, Qiao YS, Zhang Z, Xiong AS, Yao QH (2008) Genome-wide analysis of the AP2/ERF gene family in Populus trichocarpa. Biochem Bioph Res Co 371:468–474
Acknowledgements
This work was supported by the National Natural Science Foundation of China (31670272 to XD, 31171578 to YZ, 31501331 to DZ), Heilongjiang Provincial Higher School Science and Technology Innovation Team Building Program (2011TD005), and the NEAU starting grant to XD.
Author information
Authors and Affiliations
Contributions
Yang Yu and Yanming Zhu conceived the project; Yang Yu, Xiangbo Duan, Chao Chen, Lei Cao, Xuewei Song, Zaib_un Nisa, Jiyang Yu and Qiang Li performed the experiments; Pinghui Zhu, Dan Zhu, Jianying Du, Yu Song and Huiqing Li analyzed the data; Yang Yu wrote the manuscript; Beidong Liu, Kuide Yin and Xiaodong Ding revised the manuscript.
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11103_2017_623_MOESM1_ESM.tif
Supplemental Figure 1 The transcript levels of GsERF71 in the WT and OX lines. The transcription expression level of GsERF71were analyzed by semi-quantitative RT-PCR, the Actin2 gene was used as an internal standard. Supplementary material 1 (TIF 400 KB)
11103_2017_623_MOESM2_ESM.tif
Supplemental Figure 2 Overexpression of GsERF71 in Arabidopsis enhanced KHCO3 tolerance. a The phenotype of WT and GsERF71 transgenic seedlings grown on 1/2 MS medium with 0 or 6mM KHCO3. b Quantitative evaluation of leaf opening and greening rates. Experiments were performed at least three times. The bars represent standard errors. The mean values (±SE) were based on three replicates (each with 90 seeds for each line). *P< 0.05, **P< 0.01 by Student’s t test. c-d Phenotypes and measurements of primary root length of WT and OX seedlings under normal and KHCO3 stress. All mean values (±SE) were based on three independent experiments (30 seedlings per experiment). *P< 0.05, **P< 0.01 by Student’s t test. Supplementary material 2 (TIF 8931 KB)
11103_2017_623_MOESM3_ESM.tif
Supplemental Figure 3 GsERF71 did not affect plant tolerance of high pH (KOH) stress. a The growth of WT and OX seedlings on 1/2 MS medium with pH 7.5 or 8.2. Photographs were taken 7 days after germination. b Quantitative evaluation of leaf opening and greening rates. Experiments were performed at least three times (each with 90 seeds for each line). c-d Phenotypes and measurements of primary root lengths of WT and OX seedlings under normal and high pH stress. Seven-day-old seedlings grown on 1/2 MS were transferred to new solid agar plates with pH 5.8, 7.5 and 8.2, respectively. Photographs were taken after 7d growth on the media. All values are means (±SE) from three independent experiments (30 seedlings per experiment). *P < 0.05, **P < 0.01 by Student’s t test. Supplementary material 3 (TIF 1811 KB)
11103_2017_623_MOESM4_ESM.tif
Supplemental Figure 4 Expression patterns of auxin synthesis- or transport-related genes in the roots of WT and GsERF71 transgenic Arabidopsis under alkaline stress. 7-d-old WT and GsERF71 transgenic seedlings treated with 6mM NaHCO3 for up to 5 days. The mean values were based on three biological replicates for each. *P< 0.05, **P< 0.01 by two-way ANOVA with Bonferroni posttests compared with the WT at each time point. Supplementary material 4 (TIF 34011 KB)
Rights and permissions
About this article
Cite this article
Yu, Y., Duan, X., Ding, X. et al. A novel AP2/ERF family transcription factor from Glycine soja, GsERF71, is a DNA binding protein that positively regulates alkaline stress tolerance in Arabidopsis . Plant Mol Biol 94, 509–530 (2017). https://doi.org/10.1007/s11103-017-0623-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-017-0623-7