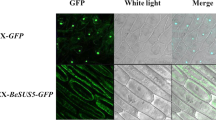
Abstract
Prior data indicated that enhanced availability of sucrose, a major product of photosynthesis in source leaves and the carbon source for secondary wall cellulose synthesis in fiber sinks, might improve fiber quality under abiotic stress conditions. To test this hypothesis, a family of transgenic cotton plants (Gossypium hirsutum cv. Coker 312 elite) was produced that over-expressed spinach sucrose-phosphate synthase (SPS) because of its role in regulation of sucrose synthesis in photosynthetic and heterotrophic tissues. A family of 12 independent transgenic lines was characterized in terms of foreign gene insertion, expression of spinach SPS, production of spinach SPS protein, and development of enhanced extractable V max SPS activity in leaf and fiber. Lines with the highest V max SPS activity were further characterized in terms of carbon partitioning and fiber quality compared to wild-type and transgenic null controls. Leaves of transgenic SPS over-expressing lines showed higher sucrose:starch ratio and partitioning of 14C to sucrose in preference to starch. In two growth chamber experiments with cool nights, ambient CO2 concentration, and limited light below the canopy, the transgenic line with the highest SPS activity in leaf and fiber had higher fiber micronaire and maturity ratio associated with greater thickness of the cellulosic secondary wall.






Similar content being viewed by others
Abbreviations
- AFIS:
-
Advanced Fiber Information System
- DPA:
-
Days post anthesis
- ELISA:
-
Enzyme-linked immunosorbent assay
- HVI:
-
High-Volume Instrumentation
- IFC %:
-
Immature fiber content expressed as a percent
- KS:
-
Kanamycin sensitive transgenic null line
- npt II:
-
Gene for neomycin phosphotransferase conferring kanamycin resistance
- PFD:
-
Photon flux density
- SFC %:
-
Short fiber content expressed as a percent
- SPS:
-
Sucrose phosphate synthase
- SPS+:
-
Transgenic cotton lines over-expressing spinach sucrose phosphate synthase
- T0-4:
-
Generations of transgenic plants, with T0 being primary transformants and numbers representing further seed-propagated generations
- WT:
-
Wild-type
References
Amor Y, Haigler CH, Johnson S, Wainscott M, Delmer DP (1995) A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc Natl Acad Sci USA 92:9353–9357
Babb VM, Haigler CH (2001) Sucrose-phosphate synthase activity rises in correlation with high-rate cellulose synthesis in three heterotrophic systems. Plant Physiol 127:1234–1242
Basra AS (ed) (1999) Cotton fibers: developmental biology, quality improvement, and textile processing. The Haworth Press, New York, pp 387
Baxter CJ, Foyer DH, Turner J, Rolfe SA, Quick WP (2003) Elevated sucrose-phosphate synthase activity in transgenic tobacco sustains photosynthesis in older leaves and alters development. J Exp Bot 54:1813–1820
Bradow JM, Davidonis GW (2000) Review: quantitation of fiber quality and the cotton production-processing interface: a physiologist’s perspective. J Cotton Sci 4:34–64
Chen S, Hajirezaei M, Peisker M, Tschiersch H, Sonnewald U, Börnke F (2005) Decreased sucrose-6-phosphate phosphatase level in transgenic tobacco inhibits photosynthesis, alters carbohydrate partitioning, and reduces growth. Planta 221:479–492
Davidonis G, Hinojosa O (1994) Influence of seed location on cotton fiber development in planta and in vitro. Plant Sci 203:107–113
Foyer CH, Galtier N (1996) Source-sink interaction and communication in leaves. In: Zamski E, Schafer AA (eds) Photoassimilate distribution in plants and crops: source sink relationships. Marcel Dekker, New York, pp 311–340
Galtier N, Foyer CH, Huber J, Voelker TA, Huber SC (1993) Effects of elevated sucrose-phosphate synthase activity on photosynthesis, assimilate partitioning, and growth in tomato (Lycopersicon esculentum var UC82B). Plant Physiol 101:535–543
Galtier N, Foyer CH, Murchie E, Alred R, Quick P, Voelker TA, Thépenier C, Lascève BT (1995) Effects of light and atmospheric carbon dioxide enrichment on photosynthesis and carbon partitioning in the leaves of tomato (Lycopersicon esculentum L) plants over-expressing sucrose-phosphate synthase. J Exp Bot 46:1335–1344
Gipson JR (1986) Temperature effects on growth, development, and fiber properties. In: Mauney JR, Stewart JMcD (eds) Cotton physiology. The Cotton Foundation, Memphis, pp 47–56
Haigler CH, Ivanova-Datcheva M, Hogan PS, Salnikov VV, Hwang S, Martin LK, Delmer DP (2001) Carbon partitioning to cellulose synthesis. Plant Mol Biol 47:29–51
Haigler CH, Zhang D, Wilkerson CG (2005) Biotechnological improvement of cotton fiber maturity. Physiol Plant 124:285–294
Haigler CH (2007) Substrate supply for cellulose synthesis and its stress sensitivity in the cotton fiber. In: Brown RM Jr, Saxena I (eds) Cellulose molecular and structural biology. Springer, New York, pp 145–166 (in press)
Hendrix DL, Grange RI (1991) Carbon partitioning and export from mature cotton leaves. Plant Physiol 95:228–233
Hequet EF, Wyatt B, Abidi N, Thibodeaux DP (2006) Creation of a set of reference material for cotton fiber maturity measurements. Text Res J 76:576–586
Holaday AS, Chollet R (1983) Photosynthetic/photorespiratory carbon metabolism in the C3-C4 intermediate species, Moricandia arvensis and Panicum milioides. Plant Physiol 73:740–745
Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2:208–218
Hsieh YL (1999) Structural development of cotton fibers and linkages to fiber quality. In: Basra AS (ed) Cotton fibers: developmental biology, quality improvement, and textile processing. The Haworth Press, New York, pp 137–166
Huber SC, Huber JLA, McMichael RW (1992) The regulation of sucrose synthesis in leaves. In: Pollock CJ (ed) Carbon partitioning within and between organisms Environmental Plant Biology Series. Oxford, Bios Scientific publishers, pp 1–26
Huber SC, Huber JL (1996) Role and regulation of sucrose-phosphate synthase in higher plants. Annu Rev Plant Physiol Plant Mol Biol 47:431–444
Im KH (2004) Expression of sucrose-phosphate synthase (SPS) in non-photosynthetic tissues of maize. Mol Cells 17:404–409
John ME (2000) Cotton fiber-specific promoters, US Patent #6,096,950; Table 7, p 36
King SP (1997) Carbon and nitrogen partitioning in canola (Brassica napus L). PhD thesis, Australian National University, Australia, 114–147
Klein RR, Crafts-Brandner SJ, Salvucci ME (1993) Cloning and developmental expression of the sucrose-phosphate synthase gene from spinach. Planta 190:498–510
Lawrence C, Holaday AS (2000) Effects of mild night chilling on respiration of expanding cotton leaves. Plant Sci 157:233–244
Lunn JE, Gillespie VJ, Furbank RT (2003) Expression of a cyanobacterial sucrose-phosphate synthase from Synechocstis sp. PCC 6803 in transgenic plants. J Exp Bot 54:223–237
Lunn JE, MacRae E (2003) New complexities in the synthesis of sucrose. Curr Opin Plant Biol 6:208–214
Manley BFJ (ed) (1997) Randomization, bootstrap and Monte Carlo methods in Biology, 2nd edn. Chapman and Hall, London
Martin LK, Haigler CH (2004) Cool temperature hinders flux from glucose to sucrose during cellulose synthesis in secondary wall stage cotton fibers. Cellulose 11:339–349
McBride KE, Summerfelt KR (1990) Improved binary vectors for Agrobacterium-mediated plant transformation. Plant Mol Biol 14:269–276
Murchie EH, Sarrobert C, Contard P, Betsche T, Foyer CH, Galtier N (1999) Over-expression of sucrose-phosphate synthase in tomato plants grown with CO2 enrichment leads to decreased foliar carbohydrate accumulation relative to untransformed controls. Plant Physiol Biochem 37:251–260
Ono K, Ishimaru K, Aoki N, Takahashi S, Ozawa K, Ohkawa Y, Ohsugi R (1999) Characterization of a maize sucrose-phosphate synthase protein and its effect on carbon partitioning in trnasgenic rice plants. Plant Prod Sci 2:172–177
Pettigrew WT (1994) Source-to-sink manipulation effects on cotton fiber quality. Agron J 87:947–952
Pierce FT, Lord E (1939) The fineness and maturity of cotton. J Text Inst 30:T173–T210
Restrep MA, Freed DD, Carrington JC (1990) Nuclear transport of plant potyviral proteins. Plant Cell 2:987–998
Roberts EM, Nunna RR, Huang JY, Trolinder NL, Haigler CH (1992) Effects of cycling temperatures on fiber metabolism in cultured cotton ovules. Plant Physiol 100:979–986
Sambrook J, Russel DW (2001) Molecular cloning, a laboratory manual 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Schubert AM, Benedict CR, Kohel RJ (1986) Carbohydrate distribution in bolls. In: Mauney JR, Stewart McDJ (eds) Cotton physiology, Cotton Foundation, Memphis, pp 311–324
Signora L, Galtier N, SkØt L, Hélène L, Foyer CH (1998) Over-expression of sucrose-phosphate synthase in Arabidopsis thaliana results in increased foliar sucrose/starch ratios and favours decreased foliar carbohydrate accumulation in plants after prolonged growth with CO2 enrichment. J Exp Bot 49:669–680
Singh B, Haley L, Nightengale J, Kang WH, Haigler CH, Holaday AS (2005) Long-term night chilling of cotton, Gossypium hirsutum, does not result in reduced CO2 assimilation. Func Plant Biol 32:655–666
Stitt M (1989) Control analysis of photosynthetic sucrose synthesis: assignment of elasticity coefficients and flux-control coefficients to the cytosolic fructose 1,6-bisphosphatase and sucrose-phosphate synthase. Phil Trans R Soc Lond B 323:327–338
Stitt M (1990) Fructose-2,6-bisphosphate as a regulatory molecule in plants. Annu Rev Plant Physiol Plant Mol Biol 41:153–185
Tarczynski MC, Byrne DN, Miller WB (1992) High performance liquid chromatography analysis of carbohydrates of cotton-phloem sap and of honeydew produced by Bemisia tabaci feeding on cotton. Plant Physiol 98:753–756
Toroser D, Athwal GS, Huber SC (1998) Site-specific regulatory interaction between spinach leaf sucrose-phosphate synthase and 14-3-3 proteins. FEBS Lett 435:110–114
Toroser D, McMichael R Jr, Krause KP, Kurreck J, Sonnewald U, Stitt M, Huber SC (1999) Site-directed mutagenesis of serine 158 demonstrates its role in spinach leaf sucrose-phosphate synthase modulation. Plant J 17:407–413
Tummala J (1996) Response of sucrose-phosphate synthase activity to cool temperatures in cotton. MS thesis, Texas Tech University, Lubbock, TX, USA
Umbeck P, Swain W, Yang NS (1989) Inheritance and expression of genes for kanamycin and chloramphenicol resistance in transgenic cotton plants. Crop Sci 29:196–201
Updegraff DM (1969) Semi-micro determination of cellulose in biological materials. Anal Biochem 32:420–424
Walker JL, Huber SC (1989) Puification and preliminary characterization of sucrose-phosphate synthase using monoclonal antibodies. Plant Physiol 89:518–524
Warner DA, Holaday AS, Burke JJ (1995) Response of carbon metabolism to night temperatures in cotton. Agron J 87:1193–1197
Wilkins TA, Wan CY, Lu CC (1994) Ancient origin of the vacuolar H+-ATPase 69-kilodalton catalytic subunit superfamily. Theor Appl Genet 89:514–524
Wilkins TA, Smart LB (1996) Isolation of RNA from plant tissue. In: Krieg A (ed) Laboratory guide to RNA: isolation, analysis, and synthesis, Wiley Liss, New York, pp 21–40
Winter H, Huber SC (2000) Regulation of sucrose metabolism in higher plants: Localization and regulation of activity of key enzymes. Crit Rev Plant Sci 19:31–67
Worrell AC, Bruneau JM, Summerfelt K, Boersig M, Voelker T (1991) Expression of maize sucrose-phosphate synthase in tomato alters carbohydrate partitioning. Plant Cell 3:1121–1130
Xu B, Huang Y (2004) Image Analysis for Cotton Fibers, Part II: Cross-Sectional Measurement. Textile Res J 74:409–416
Acknowledgments
Two anonymous reviewers are thanked for helpful comments. For providing materials, we thank Drs. Steve Huber (antibody to spinach SPS), Norma Trolinder (seeds of elite highly regenerable selections of G.h. cv Coker 312), and Hong Zhang (fragment of Arabidopsis 18S ribosomal RNA gene). For technical advice or assistance we thank: Norma Trolinder (training in cotton regeneration), Cory Reed and Michelle Morales (assistance with statistical analyses), Robert Wright (suggestions on DNA blotting protocol), and Tahan Jaradat, Nagurar Srinivas, and Melody Wainscott (preliminary analysis of SPS+ plant lines). FIAS software was developed by Dr. B. Xu (Univ. Texas-Austin). For funding and/or in-kind support of this work, we thank Cotton Incorporated Cary NC, the Texas Advanced Research, Technology, and Technology Development and Transfer Programs, the Phytotron of Duke University, the Texas Food and Fiber Commission, and Bayer CropScience.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haigler, C.H., Singh, B., Zhang, D. et al. Transgenic cotton over-producing spinach sucrose phosphate synthase showed enhanced leaf sucrose synthesis and improved fiber quality under controlled environmental conditions. Plant Mol Biol 63, 815–832 (2007). https://doi.org/10.1007/s11103-006-9127-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11103-006-9127-6