Abstract
Purpose
The burden of chronic daily subcutaneous administration of pegvisomant on adherence has not been previously studied. This study was aimed to determine the adherence to pegvisomant treatment in acromegaly patients in the real-world clinical practice setting in Spain.
Methods
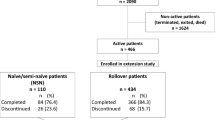
Multicenter, observational, descriptive, cross-sectional study in patients with acromegaly treated with pegvisomant for at least 12 months. Patient adherence was indirectly determined by Batalla and Haynes-Sackett questionnaires and directly by prescription record review. Additionally, treatment satisfaction was assessed by the Treatment Satisfaction with Medicines Questionnaire (SATMED-Q) and treatment convenience by an ad-hoc Pegvisomant questionnaire. Errors in reconstitution and administration process were determined by direct observation.
Results
108 patients were included in the analysis. Rates of adherence varied from 60.7 to 92.1% and did not correlate with disease control. Older patient age and alternative schedules other than daily pegvisomant dosing were associated with lower adherence. Treatment satisfaction and convenience was high, with a mean (SD) total SATMED-Q score of 74.6 ± 15.4 over 100 and a total ad-hoc Pegvisomant questionnaire score of 71.2 ± 15.2 over 100. 34.3% of patients made mistakes during the reconstitution /administration process.
Conclusions
Patient adherence to pegvisomant was high (60.7–92.1%), but more than a third of the patients in the study made mistakes during the administration process, with a potential impact on disease control. Besides dosing compliance, correct administration of medication should be carefully assessed in these patients.





Similar content being viewed by others
References
Melmed S, Colao A, Barkan A, Molitch M, Grossman AB, Kleinberg D et al (2009) Guidelines for acromegaly management: an update. J Clin Endocrinol Metab 94:1509–1517
Holdaway IM, Rajasoorya C (1999) Epidemiology of acromegaly. Pituitary 2:29–41
Lavrentaki A, Paluzzi A, Wass JA, Karavitaki N (2017) Epidemiology of acromegaly: review of population studies. Pituitary 20:4–9
Sesmilo G (2013) Epidemiology of acromegaly in Spain. Endocrinol Nutr 60:470–474
Lugo G, Pena L, Cordido F. Clinical manifestations and diagnosis of acromegaly. Int J Endocrinol. 2012,540398 (2012)
Katznelson L, Laws ER Jr, Melmed S, Molitch ME, Murad MH, Utz A et al (2014) Acromegaly: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 99:3933–3951
Giustina A, Arnaldi G, Bogazzi F, Cannavo S, Colao A, De ML et al (2017) Pegvisomant in acromegaly: an update. J Endocrinol Invest 40:577–589
van der Lely AJ, Hutson RK, Trainer PJ, Besser GM, Barkan AL, Katznelson L et al (2001) Long-term treatment of acromegaly with pegvisomant, a growth hormone receptor antagonist. Lancet 358:1754–1759
Trainer PJ, Drake WM, Katznelson L, Freda PU, Herman-Bonert V, van der Lely AJ et al (2000) Treatment of acromegaly with the growth hormone-receptor antagonist pegvisomant. N Engl J Med 342:1171–1177
van der Lely AJ, Biller BM, Brue T, Buchfelder M, Ghigo E, Gomez R et al (2012) Long-term safety of pegvisomant in patients with acromegaly: comprehensive review of 1288 subjects in ACROSTUDY. J Clin Endocrinol Metab 97:1589–1597
Bernabeu I, Pico A, Venegas E, Aller J, Alvarez-Escola C, Garcia-Arnes JA et al (2016) Safety of long-term treatment with Pegvisomant: analysis of Spanish patients included in global ACROSTUDY. Pituitary 19:127–137
Schofl C, Grussendorf M, Honegger J, Tonjes A, Thyroke-Gronostay D, Mayr B et al (2015) Failure to achieve disease control in acromegaly: cause analysis by a registry-based survey. Eur J Endocrinol 172:351–356
Dunbar-Jacob J, Mortimer-Stephens MK (2001) Treatment adherence in chronic disease. J Clin Epidemiol 54(Suppl 1,S):57–60
Simpson SH, Eurich DT, Majumdar SR, Padwal RS, Tsuyuki RT, Varney J et al (2006) A meta-analysis of the association between adherence to drug therapy and mortality. BMJ 333:15
WHO (2003) Adherence to long-term therapies: evidence for action. World Health Organization, Geneva. http://www.who.int/chp/knowledge/publications/adherence_report_fin.pdf?ua=1
Joosten EA, DeFuentes-Merillas L, de Weert GH, Sensky T, van der Staak CP, de Jong CA (2008) Systematic review of the effects of shared decision-making on patient satisfaction, treatment adherence and health status. Psychother Psychosom 77:219–226
Saarti S, Hajj A, Karam L, Jabbour H, Sarkis A, El ON et al (2016) Association between adherence, treatment satisfaction and illness perception in hypertensive patients. J Hum Hypertens 30:341–345
Witek P, Mucha S, Ruchala M (2016) Patient satisfaction and preferences of lanreotide Autogel treatment in acromegaly. Endokrynol Pol 67:572–579
Brody DS, Miller SM, Lerman CE, Smith DG, Caputo GC (1989) Patient perception of involvement in medical care: relationship to illness attitudes and outcomes. J Gen Intern Med 4:506–511
Kepicoglu H, Hatipoglu E, Bulut I, Darici E, Hizli N, Kadioglu P (2014) Impact of treatment satisfaction on quality of life of patients with acromegaly. Pituitary 17:557–563
Batalla A, Blanquer R, Ciurana M, García M, Jordi E, Pérez A (1984) Cumplimiento de la prescripción farmacológica en pacientes hipertensos. Aten Primaria 1:185–191
Barnestein-Fonseca P, Leiva-Fernandez J, Vidal-Espana F, Garcia-Ruiz A, Prados-Torres D, Leiva-Fernandez F (2011) Is it possible to diagnose the therapeutic adherence of patients with COPD in clinical practice? A cohort study. BMC Pulm Med 11:6
Haynes RB, Taylor DW, Sackett DL, Gibson ES, Bernholz CD, Mukherjee J. Can simple clinical measurements detect patient noncompliance? Hypertension. 2,757–764 (1980)
Ruiz MA, Pardo A, Rejas J, Soto J, Villasante F, Aranguren JL (2008) Development and validation of the “Treatment Satisfaction with Medicines Questionnaire” (SATMED-Q). Value Health 11:913–926
Haynes RB, Taylor DL, Sackett D (1979) Compliance in Health Care. John Hopkins University Press, Baltimore
Pineiro F, Gil V, Donis M, Orozco D, Pastor R, Merino J (1997) The validity of 6 indirect methods for assessing drug treatment compliance in arterial hypertension. Aten Primaria 19(372-4):376
Nazir SU, Hassali MA, Saleem F, Bashir S, Aljadhey H (2016) Association between diabetes-related knowledge and medication adherence: results from cross-sectional analysis. Altern Ther Health Med 22:8–13
Slabaugh SL, Bouchard JR, Li Y, Baltz JC, Meah YA, Moretz DC (2015) Characteristics relating to adherence and persistence to basal insulin regimens among elderly insulin-naive patients with type 2 diabetes: pre-filled pens versus vials/syringes. Adv Ther 32:1206–1221
Freda PU, Gordon MB, Kelepouris N, Jonsson P, Koltowska-Haggstrom M, van der Lely AJ (2015) Long-term treatment with pegvisomant as monotherapy in patients with acromegaly: experience from ACROSTUDY. Endocr Pract 21:264–274
McDowell SE, Mt-Isa S, Ashby D, Ferner RE (2010) Where errors occur in the preparation and administration of intravenous medicines: a systematic review and Bayesian analysis. Qual Saf Health Care 19:341–345
Ramos-Levi AM, Bernabeu I, Alvarez-Escola C, Aller J, Lucas T de (2016) MP et al. Long-term treatment with pegvisomant for acromegaly: a 10-year experience. Clin Endocrinol (Oxf) 84:540–550
Strasburger CJ, Karavitaki N, Stormann S, Trainer PJ, Kreitschmann-Andermahr I, Droste M et al (2016) Patient-reported outcomes of parenteral somatostatin analogue injections in 195 patients with acromegaly. Eur J Endocrinol 174:355–362
Acknowledgements
The authors thank all the investigators of PEGASO study and participant patients at Hospital Universitario La Paz (Dr. Cristina Álvarez), Hospital Universitario La Princesa (Dr. Ana Mª Ramos), Hospital Regional Universitario Carlos Haya (Dr. Juan Antonio García-Arnés), Hospital Universitario Virgen del Rocío (Dr. Eva Venegas), Hospital Universitario y Politécnico La Fe (Dr. Rosa Cámara), Hospital Universitario La Ribera (Dr. Carmen Fajardo), Hospital General Universitario de Valencia (Dr. Juan Carlos Ferrer), Hospital General Universitario de Alicante (Dr. Antonio Picó), Hospital Universitario Vall d’Hebrón (Dr. Jordi Mesa), Hospital Universitario de Bellvitge (Dr. Carles Villabona), Hospital Universitario Puerta de Hierro (Dr. Javier Aller), Hospital Universitario de Getafe (Dr. Isabel Pavón), Hospital General Universitario Gregorio Marañón (Dr. Rogelio García), Hospital Universitario Marqués de Valdecilla (Dr. Fernando Pazos), Hospital Universitario de Cruces (Dra. Sonia Gaztambide), Hospital Universitario Basurto (Dr. Miguel Paja), Hospital Universitario Miguel Servet (Dr. Javier Acha), Complejo Hospitalario Universitario Santiago (Dr. Ignacio Bernabéu), Complejo Hospitalario Universitario A Coruña (Dr. Fernando Cordido).
Funding
This study was funded by Pfizer (Spain). Medical writing support was provided by Esther Tapia PhD as a freelance medical writer and was funded by Pfizer (Spain).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
NM and LS-C are employees of Pfizer (Spain). MLS works for Pfizer (Spain). RC, EV, JAG-A, FC and JA have received compensation from Pfizer (Spain) for their participation as investigators of PEGASO study. RC has received compensation from Pfizer (Spain) for their participation as research coordinator of PEGASO study. RC, EV, FC and JA have received speaker honoraria from Pfizer (Spain).
Ethical approval
The study was approved by the Clinical Research Ethics Committee of every participant site. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committees and with the 1964 Helsinki declaration and its later amendments.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cámara, R., Venegas, E., García-Arnés, J.A. et al. Treatment adherence to pegvisomant in patients with acromegaly in Spain: PEGASO study. Pituitary 22, 137–145 (2019). https://doi.org/10.1007/s11102-019-00943-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-019-00943-1