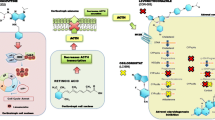
Abstract
Transsphenoidal surgery remains the first line therapy in Cushing’s disease, but a large number of patients will not be cured or disease will recur over time. Repeat pituitary surgery, bilateral adrenalectomy, and radiation have limitations with respect to efficacy and/or side effects. Therefore, there is a clear need for an effective medical treatment. The studies reviewed here suggest a role for pituitary-directed therapies, applying multireceptor ligand somatostatin analogs like pasireotide or second-generation dopamine agonists. Retinoic acid has been also studied in a small prospective study. These compounds target ACTH-secretion at the pituitary level and possibly inhibit corticotrope proliferation. Specific side effects of these compounds need to be considered, especially when used as long-term therapy. These novel approaches could provide options for treatment of patients in whom surgery has failed or is not possible, and while awaiting effects of radiation therapy. Preoperative use to decrease cortisol excess, potentially reducing perioperative complications, needs to be further studied.


Similar content being viewed by others
References
Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, Buchfelder M, Colao A, Hermus AR, Hofland LJ, Klibanski A, Lacroix A, Lindsay JR, Newell-Price J, Nieman LK, Petersenn S, Sonino N, Stalla GK, Swearingen B, Vance ML, Wass JA, Boscaro M (2008) Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 93(7):2454–2462. doi:10.1210/jc.2007-2734
Fleseriu M, Petersenn S (2012) Medical management of Cushing’s disease: what is the future? Pituitary 15(3):330–341. doi:10.1007/s11102-012-0397-5
Stewart PM, Petersenn S (2009) Rationale for treatment and therapeutic options in Cushing’s disease. Best Pract Res Clin Endocrinol Metab 23(Suppl 1):S15–S22. doi:10.1016/S1521-690X(09)70004-1
Nieman LK (2013) Update in the medical therapy of Cushing’s disease. Curr Opin Endocrinol Diabetes Obes 20(4):330–334. doi:10.1097/MED.0b013e3283631809
Fleseriu M, Petersenn S (2013) New avenues in the medical treatment of Cushing’s disease: corticotroph tumor targeted therapy. J Neurooncol 114(1):1–11. doi:10.1007/s11060-013-1151-1
de Bruin C, Feelders RA, Lamberts SW, Hofland LJ (2009) Somatostatin and dopamine receptors as targets for medical treatment of Cushing’s syndrome. Rev Endocr Metab Disord 10(2):91–102. doi:10.1007/s11154-008-9082-4
Pecori-Giraldi F, Ambrogio AG, Andrioli M, Sanguin F, Karamouzis I, Corsello SM, Scaroni C, Arvat E, Pontecorvi A, Cavagnini F (2012) Potential role for retinoic acid in patients with Cushing’s disease. J Clin Endocrinol Metab 97(10):3577–3583. doi:10.1210/jc.2012-2328
Stefaneanu L, Kovacs K, Horvath E, Buchfelder M, Fahlbusch R, Lancranjan L (2001) Dopamine D2 receptor gene expression in human adenohypophysial adenomas. Endocrine 14(3):329–336
Adams EF, Ashby MJ, Brown SM, White MC, Mashiter K (1981) Bromocriptine suppresses ACTH secretion from human pituitary tumour cells in culture by a dopaminergic mechanism. Clin Endocrinol (Oxf) 15(5):479–484
Yin D, Kondo S, Takeuchi J, Morimura T (1994) Induction of apoptosis in murine ACTH-secreting pituitary adenoma cells by bromocriptine. FEBS Lett 339(1–2):73–75
Miller JW, Crapo L (1993) The medical treatment of Cushing’s syndrome. Endocr Rev 14(4):443–458
Hale AC, Coates PJ, Doniach I, Howlett TA, Grossman A, Rees LH, Besser GM (1988) A bromocriptine-responsive corticotroph adenoma secreting alpha-MSH in a patient with Cushing’s disease. Clin Endocrinol (Oxf) 28(2):215–223
Koppeschaar HP, Croughs RJ, Thijssen JH, Schwarz F (1986) Response to neurotransmitter modulating drugs in patients with Cushing’s disease. Clin Endocrinol (Oxf) 25(6):661–667
Pivonello R, Ferone D, de Herder WW, Kros JM, De Caro ML, Arvigo M, Annunziato L, Lombardi G, Colao A, Hofland LJ, Lamberts SW (2004) Dopamine receptor expression and function in corticotroph pituitary tumors. J Clin Endocrinol Metab 89(5):2452–2462
Pivonello R, De Martino MC, Cappabianca P, De Leo M, Faggiano A, Lombardi G, Hofland LJ, Lamberts SW, Colao A (2009) The medical treatment of Cushing’s disease: effectiveness of chronic treatment with the dopamine agonist cabergoline in patients unsuccessfully treated by surgery. J Clin Endocrinol Metab 94(1):223–230. doi:10.1210/jc.2008-1533
Godbout A, Manavela M, Danilowicz K, Beauregard H, Bruno OD, Lacroix A (2010) Cabergoline monotherapy in the long-term treatment of Cushing’s disease. Eur J Endocrinol 163(5):709–716. doi:10.1530/EJE-10-0382
Manavela MP, Danilowicz K, Bruno OD (2012) Macrocorticotropinoma shrinkage and control of hypercortisolism under long-term cabergoline therapy: case report. Pituitary 15(Suppl 1):33–36. doi:10.1007/s11102-011-0309-0
Ahmed A, Furqan S, Islam N (2012) Disappearance of pituitary macro adenoma with combination of ketoconazole and cabergoline treatment: an unusual case of Cushing’s syndrome with interesting findings. BMJ Case Rep. doi:10.1136/bcr.03.2012.6025
Lindsay JR, Nieman LK (2005) The hypothalamic-pituitary-adrenal axis in pregnancy: challenges in disease detection and treatment. Endocr Rev 26(6):775–799. doi:10.1210/er.2004-0025
Lindsay JR, Jonklaas J, Oldfield EH, Nieman LK (2005) Cushing’s syndrome during pregnancy: personal experience and review of the literature. J Clin Endocrinol Metab 90(5):3077–3083. doi:10.1210/jc.2004-2361
Woo I, Ehsanipoor RM (2013) Cabergoline therapy for Cushing disease throughout pregnancy. Obstet Gynecol 122(2 Pt 2):485–487. doi:10.1097/AOG.0b013e31829e398a
Schade R, Andersohn F, Suissa S, Haverkamp W, Garbe E (2007) Dopamine agonists and the risk of cardiac-valve regurgitation. N Engl J Med 356(1):29–38. doi:10.1056/NEJMoa062222
Zanettini R, Antonini A, Gatto G, Gentile R, Tesei S, Pezzoli G (2007) Valvular heart disease and the use of dopamine agonists for Parkinson’s disease. N Engl J Med 356(1):39–46. doi:10.1056/NEJMoa054830
Boschetti M, Gatto F, Arvigo M, Esposito D, Rebora A, Talco M, Albertelli M, Nazzari E, Goglia U, Minuto F, Ferone D (2010) Role of dopamine receptors in normal and tumoral pituitary corticotropic cells and adrenal cells. Neuroendocrinology 92(Suppl 1):17–22. doi:10.1159/000314293
Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G (2002) SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol 146(5):707–716 doi:10.1530/eje.0.1460707
Schmid HA, Schoeffter P (2004) Functional activity of the multiligand analog SOM230 at human recombinant somatostatin receptor subtypes supports its usefulness in neuroendocrine tumors. Neuroendocrinology 80(Suppl 1):47–50. doi:10.1159/000080741
Hofland LJ, van der Hoek J, Feelders R, van Aken MO, van Koetsveld PM, Waaijers M, Sprij-Mooij D, Bruns C, Weckbecker G, de Herder WW, Beckers A, Lamberts SW (2005) The multi-ligand somatostatin analogue SOM230 inhibits ACTH secretion by cultured human corticotroph adenomas via somatostatin receptor type 5. Eur J Endocrinol 152(4):645–654. doi:10.1530/eje.1.01876
van der Hoek J, Waaijers M, van Koetsveld PM, Sprij-Mooij D, Feelders RA, Schmid HA, Schoeffter P, Hoyer D, Cervia D, Taylor JE, Culler MD, Lamberts SW, Hofland LJ (2005) Distinct functional properties of native somatostatin receptor subtype 5 compared with subtype 2 in the regulation of ACTH release by corticotroph tumor cells. Am J Physiol Endocrinol Metab 289(2):E278–E287. doi:10.1152/ajpendo.00004.2005
Batista DL, Zhang X, Gejman R, Ansell PJ, Zhou Y, Johnson SA, Swearingen B, Hedley-Whyte ET, Stratakis CA, Klibanski A (2006) The effects of SOM230 on cell proliferation and adrenocorticotropin secretion in human corticotroph pituitary adenomas. J Clin Endocrinol Metab 91(11):4482–4488. doi:10.1210/jc.2006-1245
Silva AP, Bethmann K, Raulf F, Schmid HA (2005) Regulation of ghrelin secretion by somatostatin analogs in rats. Eur J Endocrinol 152(6):887–894. doi:10.1530/eje.1.01914
Ben-Shlomo A, Schmid H, Wawrowsky K, Pichurin O, Hubina E, Chesnokova V, Liu NA, Culler M, Melmed S (2009) Differential ligand-mediated pituitary somatostatin receptor subtype signaling: implications for corticotroph tumor therapy. J Clin Endocrinol Metab 94(11):4342–4350. doi:10.1210/jc.2009-1311
Boscaro M, Ludlam WH, Atkinson B, Glusman JE, Petersenn S, Reincke M, Snyder P, Tabarin A, Biller BM, Findling J, Melmed S, Darby CH, Hu K, Wang Y, Freda PU, Grossman AB, Frohman LA, Bertherat J (2009) Treatment of pituitary-dependent Cushing’s disease with the multireceptor ligand somatostatin analog pasireotide (SOM230): a multicenter, phase II trial. J Clin Endocrinol Metab 94(1):115–122. doi:10.1210/jc.2008-1008
Boscaro M, Bertherat J, Findling J, Fleseriu M, Atkinson AB, Petersenn S, Schopohl J, Snyder P, Hughes G, Trovato A, Hu K, Maldonado M, Biller BM (2014) Extended treatment of Cushing’s disease with pasireotide: results from a 2-year, Phase II study. Pituitary 17(4):320–326. doi:10.1007/s11102-013-0503-3
Colao A, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, Schoenherr U, Mills D, Salgado LR, Biller BMK (2012) A 12-month phase 3 study of pasireotide in Cushing’s Disease. N Engl J Med 366(10):914–924. doi:10.1056/NEJMoa1105743
Webb SM, Ware JE, Forsythe A, Yang M, Badia X, Nelson LM, Signorovitch JE, McLeod L, Maldonado M, Zgliczynski W, de Block C, Portocarrero-Ortiz L, Gadelha M (2014) Treatment effectiveness of pasireotide on health-related quality of life in patients with Cushing’s disease. Eur J Endocrinol 171(1):89–98. doi:10.1530/EJE-13-1013
Pivonello R, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, Trovato A, Hughes G, Salgado LR, Lacroix A, Schopohl J, Biller BM, Pasireotide BSG (2014) Pasireotide treatment significantly improves clinical signs and symptoms in patients with Cushing’s disease: results from a Phase III study. Clin Endocrinol (Oxf) 81(3):408–417. doi:10.1111/cen.12431
van der Pas R, van Esch JH, de Bruin C, Danser AH, Pereira AM, Zelissen PM, Netea-Maier R, Sprij-Mooij DM, van den Berg-Garrelds IM, van Schaik RH, Lamberts SW, van den Meiracker AH, Hofland LJ, Feelders RA (2014) Cushing’s disease and hypertension: in vivo and in vitro study of the role of the renin-angiotensin-aldosterone system and effects of medical therapy. Eur J Endocrinol 170(2):181–191. doi:10.1530/EJE-13-0477
MacKenzie-Feder J, Bourdeau I, Vallette S, Beauregard H, Ste-Marie LG, Lacroix A (2014) pasireotide monotherapy in Cushing’s disease: a single-centre experience with 5-year extension of phase III Trial. Pituitary 17(6):519–529. doi:10.1007/s11102-013-0539-4
Cukier K, Tewari R, Kurth F, Schmid HA, Lai C, Torpy DJ (2009) Significant response to pasireotide (SOM230) in the treatment of a patient with persistent, refractory Cushing’s disease. Clin Endocrinol (Oxf) 71(2):305–307. doi:10.1111/j.1365-2265.2008.03486.x
Shimon I, Rot L, Inbar E (2012) Pituitary-directed medical therapy with pasireotide for a corticotroph macroadenoma: pituitary volume reduction and literature review. Pituitary 15(4):608–613. doi:10.1007/s11102-012-0427-3
Lu L, Duan L, Jin Z, Lu Z, Gu F (2013) Effective long-term treatment of Cushing’s disease with pasireotide: a case report. Endocr Pract 19(4):e92–e96. doi:10.4158/EP12296.CR
Trementino L, Cardinaletti M, Concettoni C, Marcelli G, Boscaro M, Arnaldi G (2014) Up-to 5-year efficacy of pasireotide in a patient with Cushing’s disease and pre-existing diabetes: literature review and clinical practice considerations. Pituitary. doi:10.1007/s11102-014-0582-9
Libe R, Groussin L, Bertherat J (2012) Pasireotide in Cushing’s disease. N Engl J Med 366(22):2134–2135. doi:10.1056/NEJMc1204078#SA2
Katznelson L (2013) Sustained improvements in plasma ACTH and clinical status in a patient with Nelson’s syndrome treated with pasireotide LAR, a multireceptor somatostatin analog. J Clin Endocrinol Metab 98(5):1803–1807. doi:10.1210/jc.2013-1497
Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, Sen K, Salgado LR, Colao A, Biller BM, Pasireotide BSG (2014) High variability in baseline urinary free cortisol values in patients with Cushing’s disease. Clin Endocrinol (Oxf) 80(2):261–269. doi:10.1111/cen.12259
Carroll T, Raff H, Findling JW (2008) Late-night salivary cortisol measurement in the diagnosis of Cushing’s syndrome. Nat Clin Pract Endocrinol Metab 4(6):344–350. doi:10.1038/ncpendmet0837
Deutschbein T, Broecker-Preuss M, Flitsch J, Jaeger A, Althoff R, Walz MK, Mann K, Petersenn S (2012) Salivary cortisol as a diagnostic tool for Cushing’s syndrome and adrenal insufficiency: improved screening by an automatic immunoassay. Eur J Endocrinol 166(4):613–618. doi:10.1530/EJE-11-0945
Trementino L, Cardinaletti M, Concettoni C, Marcelli G, Polenta B, Spinello M, Boscaro M, Arnaldi G (2014) Salivary cortisol is a useful tool to assess the early response to pasireotide in patients with Cushing’s disease. Pituitary. doi:10.1007/s11102-014-0557-x
Paez-Pereda M, Kovalovsky D, Hopfner U, Theodoropoulou M, Pagotto U, Uhl E, Losa M, Stalla J, Grubler Y, Missale C, Arzt E, Stalla GK (2001) Retinoic acid prevents experimental Cushing syndrome. J Clin Invest 108(8):1123–1131. doi:10.1172/JCI11098
Castillo V, Giacomini D, Paez-Pereda M, Stalla J, Labeur M, Theodoropoulou M, Holsboer F, Grossman AB, Stalla GK, Arzt E (2006) Retinoic acid as a novel medical therapy for Cushing’s disease in dogs. Endocrinology 147(9):4438–4444. doi:10.1210/en.2006-0414
Conflict of interest
S. Petersenn—presentation at workshops organized by Novartis and Ipsen, member of advisory boards for Ipsen, Novartis, Pfizer, and Roche. M. Fleseriu—research grants to Oregon Health & Science University from Cortendo, Ipsen, Novartis Opko, and Pfizer; ad-hoc scientific consultant/advisor Genentech, Novartis, Xoma.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Petersenn, S., Fleseriu, M. Pituitary-directed medical therapy in Cushing’s disease. Pituitary 18, 238–244 (2015). https://doi.org/10.1007/s11102-015-0639-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-015-0639-4