Abstract
Purpose
Late night salivary cortisol (LNSC) is useful for diagnosing hypercortisolism and monitoring patients with Cushing’s disease (CD) following pituitary surgery. It may also be a better index of cortisol secretion than serum cortisol or urinary free cortisol (UFC). No data regarding the role of LNSC in the early monitoring of patients with CD receiving drug therapy has been published. We investigated the value of LNSC in monitoring the short-term efficacy of pasireotide.
Methods
Seven patients who were enrolled in a phase II study investigating the efficacy of pasireotide in CD (CSOM230B2208) were included in this analysis. Patients self-administered subcutaneous pasireotide 600 μg bid for 15 days. LNSC and UFC levels were assessed at baseline and day 15.
Results
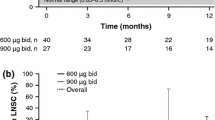
At baseline, all patients had elevated LNSC which was correlated significantly with UFC levels (r = 0.97, P = .0002). At day 15, LNSC was reduced in six patients. LNSC decreases were observed from day 1 (−20 %) and persisted until day 15 (overall mean reduction from baseline −51 %), with the greatest decrease on day 5 (−58 %). At day 15, UFC levels were decreased in all patients and normalized in one that restored also salivary cortisol rhythm.
Conclusions
In patients with CD, pasireotide rapidly reduced and normalized both UFC and LNSC levels. LNSC may be a simple, non-invasive biomarker to assess the early response to pasireotide, particularly in determining whether cortisol rhythm is normalized in patients with normalized UFC levels. Further studies are warranted.



Similar content being viewed by others
References
Arnaldi G, Angeli A, Atkinson AB, Bertagna X, Cavagnini F, Chrousos GP, Fava GA, Findling JW, Gaillard RC, Grossman AB, Kola B, Lacroix A, Mancini T, Mantero F, Newell-Price J, Nieman LK, Sonino N, Vance ML, Giustina A, Boscaro M (2003) Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 88(12):5593–5602
Arnaldi G, Mancini T, Tirabassi G, Trementino L, Boscaro M (2012) Advances in the epidemiology, pathogenesis, and management of Cushing’s syndrome complications. J Endocrinol Invest 35(4):434–448
Biller BM, Grossman AB, Stewart PM, Melmed S, Bertagna X, Bertherat J, Buchfelder M, Colao A, Hermus AR, Hofland LJ, Klibanski A, Lacroix A, Lindsay JR, Newell-Price J, Nieman LK, Petersenn S, Sonino N, Stalla GK, Swearingen B, Vance ML, Wass JA, Boscaro M (2008) Treatment of adrenocorticotropin-dependent Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 93(7):2454–2462
Hofland LJ, Lamberts SW (2003) The pathophysiological consequences of somatostatin receptor internalization and resistance. Endocr Rev 24(1):28–47
Batista DL, Zhang X, Gejman R, Ansell PJ, Zhou Y, Johnson SA, Swearingen B, Hedley-Whyte ET, Stratakis CA, Klibanski A (2006) The effects of SOM230 on cell proliferation and adrenocorticotropin secretion in human corticotroph pituitary adenomas. J Clin Endocrinol Metab 91(11):4482–4488
Bruns C, Lewis I, Briner U, Meno-Tetang G, Weckbecker G (2002) SOM230: a novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol 146(5):707–716
Hofland LJ, van der Hoek J, Feelders R, van Aken MO, van Koetsveld PM, Waaijers M, Sprij-Mooij D, Bruns C, Weckbecker G, de Herder WW, Beckers A, Lamberts SW (2005) The multi-ligand somatostatin analogue SOM230 inhibits ACTH secretion by cultured human corticotroph adenomas via somatostatin receptor type 5. Eur J Endocrinol 152(4):645–654
van der Hoek J, Waaijers M, van Koetsveld PM, Sprij-Mooij D, Feelders RA, Schmid HA, Schoeffter P, Hoyer D, Cervia D, Taylor JE, Culler MD, Lamberts SW, Hofland LJ (2005) Distinct functional properties of native somatostatin receptor subtype 5 compared with subtype 2 in the regulation of ACTH release by corticotroph tumor cells. Am J Physiol Endocrinol Metab 289(2):E278–E287
Boscaro M, Ludlam WH, Atkinson B, Glusman JE, Petersenn S, Reincke M, Snyder P, Tabarin A, Biller BM, Findling J, Melmed S, Darby CH, Hu K, Wang Y, Freda PU, Grossman AB, Frohman LA, Bertherat J (2009) Treatment of pituitary-dependent Cushing’s disease with the multireceptor ligand somatostatin analog pasireotide (SOM230): a multicenter, phase II trial. J Clin Endocrinol Metab 94(1):115–122
Colao A, Petersenn S, Newell-Price J, Findling JW, Gu F, Maldonado M, Schoenherr U, Mills D, Salgado LR, Biller BM (2012) Pasireotide B2305 study group: a 12-month phase 3 study of pasireotide in Cushing’s disease. N Engl J Med 366(10):914–924
Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM (2008) The diagnosis of Cushing’s syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 93(5):1526–1540
Papanicolaou DA, Mullen N, Kyrou I, Nieman LK (2002) Nighttime salivary cortisol: a useful test for the diagnosis of Cushing’s syndrome. J Clin Endocrinol Metab 87(10):4515–4521
Putignano P, Toja P, Dubini A, Pecori Giraldi F, Corsello SM, Cavagnini F (2003) Midnight salivary cortisol versus urinary free and midnight serum cortisol as screening tests for Cushing’s syndrome. J Clin Endocrinol Metab 88(9):4153–4157
Raff H, Raff JL, Findling JW (1998) Late-night salivary cortisol as a screening test for Cushing’s syndrome. J Clin Endocrinol Metab 83(8):2681–2686
Viardot A, Huber P, Puder JJ, Zulewski H, Keller U, Müller B (2005) Reproducibility of nighttime salivary cortisol and its use in the diagnosis of hypercortisolism compared with urinary free cortisol and overnight dexamethasone suppression test. J Clin Endocrinol Metab 90(10):5730–5736
Yaneva M, Mosnier-Pudar H, Dugué MA, Grabar S, Fulla Y, Bertagna X (2004) Midnight salivary cortisol for the initial diagnosis of Cushing’s syndrome of various causes. J Clin Endocrinol Metab 89(7):3345–3351
Raff H (2012) Cushing’s syndrome: diagnosis and surveillance using salivary cortisol. Pituitary 15(1):64–70
Carrasco CA, Coste J, Guignat L, Groussin L, Dugué MA, Gaillard S, Bertagna X, Bertherat J (2008) Midnight salivary cortisol determination for assessing the outcome of transsphenoidal surgery in Cushing’s disease. J Clin Endocrinol Metab 93(12):4728–4734
Nunes ML, Vattaut S, Corcuff JB, Rault A, Loiseau H, Gatta B, Valli N, Letenneur L, Tabarin A (2009) Late-night salivary cortisol for diagnosis of overt and subclinical Cushing’s syndrome in hospitalized and ambulatory patients. J Clin Endocrinol Metab 94(2):456–462
Jonsson BA, Malmberg B, Amilon A, Helene Garde Am, Orbaek P (2003) Determination of cortisol in human saliva using liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci 784(1):63–68
Findling JW, Raff H (2006) Cushing’s syndrome: important issues in diagnosis and management. J Clin Endocrinol Metab 91(10):3746–3753
Ceccato F, Albiger N, Reimondo G, Frigo AC, Ferasin S, Occhi G, Mantero F, Terzolo M, Scaroni C (2012) Assessment of glucocorticoid therapy with salivary cortisol in secondary adrenal insufficiency. Eur J Endocrinol 167(6):769–776
Newell-Price J, Petersenn S, Pivonello R, Findling J, Fleseriu M, Trovato A, Hughes G, Ligueros-Saylan M, Biller B (2013) Evaluation of late-night salivary cortisol during a phase III study with pasireotide in patients with Cushing’s disease [abstract]. Endocrine Abstracts 32:P853. doi:10.1530/endoabs.32.P853
Raff H (2009) Utility of salivary cortisol measurements in Cushing’s syndrome and adrenal insufficiency. J Clin Endocrinol Metab 94(10):3647–3655
Raff H, Findling JW (2003) A physiologic approach to diagnosis of the Cushing syndrome. Ann Intern Med 138:980–991
Hellhammer DH, Wüst S, Kudielka BM (2009) Salivary cortisol as a biomarker in stress research. Psychoneuroendocrinology 34:163–171
Kidambi S, Raff H, Findling JW (2007) Limitations of nocturnal salivary cortisol and urine free cortisol in the diagnosis of mild Cushing’s syndrome. Eur J Endocrinol 157(6):725–731
Terzolo M, Bovio S, Pia A, Conton PA, Reimondo G, Dall’Asta C, Bemporad D, Angeli A, Opocher G, Mannelli M, Ambrosi B, Mantero F (2005) Midnight serum cortisol as a marker of increased cardiovascular risk in patients with a clinically inapparent adrenal adenoma. Eur J Endocrinol 153(2):307–315
Oltmanns KM, Dodt B, Schultes B, Raspe HH, Schweiger U, Born J, Fehm HL, Peters A (2006) Cortisol correlates with metabolic disturbances in a population study of type 2 diabetic patients. Eur J Endocrinol 154(2):325–331
Acknowledgments
The authors would like to thank Simone Boniface of in Science Communications, Springer Healthcare, who provided editorial assistance and styling prior to submission. The authors received editorial/writing support in the preparation of this manuscript provided by in Science Communications, Springer Healthcare. This support was funded by Novartis, Italy. The authors did not receive honoraria related to the preparation of this manuscript.
Conflict of interest
MS is an employee of Novartis, the manufacturer of pasireotide. The other authors have no conflict of interest to declare. The other authors have nothing to disclose.
Author information
Authors and Affiliations
Corresponding author
Additional information
Laura Trementino and Marina Cardinaletti have contributed equally to the present work.
Clinical Trial Registration: NCT00088608.
Rights and permissions
About this article
Cite this article
Trementino, L., Cardinaletti, M., Concettoni, C. et al. Salivary cortisol is a useful tool to assess the early response to pasireotide in patients with Cushing’s disease. Pituitary 18, 60–67 (2015). https://doi.org/10.1007/s11102-014-0557-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11102-014-0557-x