Abstract
Background Liver injury has been documented independently in novel coronavirus disease 2019 (COVID-19) patients and patients treated with lopinavir-ritonavir. Objective to investigate the drug-induced liver injury associated with lopinavir-ritonavir among the patients with COVID-19. Methods We conducted a disproportionality analysis of US Food and Drug Administration Adverse Event Reporting System (FAERS) between 2020Q1 and 2021Q1 to evaluate the association between lopinavir-ritonavir and risk of drug-induced liver injury (or severe drug-induced liver injury) and calculated their reporting odds ratios (RORs) with 95% confidence intervals (CIs). Results A total of 3,425 cases of drug-induced liver injury were reported in 19,782 patients with COVID-19. The ROR for drug-induced liver injury was 2.99 (2.59–3.46), 3.16 (2.68–3.73), and 5.39 (4.63–6.26) when comparing lopinavir-ritonavir with all other drugs, hydroxychloroquine/chloroquine only, and remdesivir, respectively. For severe drug-induced liver injury, RORs for lopinavir-ritonavir provided evidence of an association compared with all other drugs (3.98; 3.15–5.05), compared with hydroxychloroquine/chloroquine only (5.33; 4.09–6.94), and compared with remdesivir (3.85; 3.03–4.89). Conclusions In the FAERS, we observed a disproportional signal for drug-induced liver injury associated with lopinavir-ritonavir in patients with COVID-19.
Similar content being viewed by others
Impacts on practice
-
COVID-19 patients treated with lopinavir-ritonavir are at increased risk of liver injury.
-
The risk of liver injury reinforces recent guidelines recommending against the use of lopinavir-ritonavir in COVID-19.
-
Further study of lopinavir-ritonavir is required to better characterize the risk factors and outcomes of liver injury in treated patients.
Introduction
The coronavirus disease 2019 (COVID-19) pandemic has become a global public health crisis. COVID-19 not only produces clusters of severe respiratory illness but also leads to multiorgan failure and death [1]. Liver injury is common in patients with COVID-19 and is more commonly observed in severe COVID-19 [2]. The mechanism by which COVID-19 may lead to elevated liver enzymes is unclear, but it may be related to several factors including direct virus-induced cytopathic effect, thromboembolic complications, and COVID-19-associated cytokine release [2]. Drug-induced liver injury is another important potential contributor [3], but data are limited regarding drug hepatoxicity in patients with COVID-19.
Lopinavir-ritonavir, a fixed-dose combination antiretroviral drug widely used for the treatment and prevention of HIV/AIDS, has also been a potential candidate for treating COVID-19 in the early pandemic [4]. Moreover, lopinavir-ritonavir is considered as an independent factor for liver injury [5]; however, liver injury associated with lopinavir-ritonavir in COVID-19 patients has not been well described.
Aim of the study
In this study, we investigated the drug-induced liver injury associated with lopinavir-ritonavir from the US Food and Drug Administration Adverse Event Reporting System (FAERS).
Ethics approval
The study requires no ethics approval.
Methods
FAERS, a publicly available spontaneous reporting system, is designed to support the FDA’s post-marketing surveillance for drug and therapeutic biologic products. The FAERS containing drug AE reports, product quality complaints, and medication error reports, is published every quarter. As of March 31 (Q1), 2021 (the most recent update date at submission), a total of 22,002,078 adverse event reports were submitted to FAERS.
In this study, we extracted data for patients with COVID-19 from FAERS between January 1, 2020 (2020 Q1) and 2021 Q1 using the COVID-19 related terms within Medical Dictionary for Regulatory Activities (MedDRA, version 23.1) (See Table 1). Our outcomes included drug-induced liver injury and severe drug-induced liver injury, which were identified by a narrow Standardized MedDRA Query (SMQ) of “drug related hepatic disorders” and by a narrow SMQ of “drug related hepatic disorders—severe events only”, respectively (definitions shown in Table 1) [6]. All drugs of interest were identified through their generic and brand names.
We conducted a disproportionality analysis and calculated reporting odds ratios (RORs) with 95% confidence intervals (CI) [7] for the following comparisons: (1) lopinavir-ritonavir versus all other drugs (all drugs except lopinavir-ritonavir used in the COVID-19 cases); (2) lopinavir-ritonavir versus hydroxychloroquine/chloroquine; (3) lopinavir-ritonavir versus remdesivir. The ROR is calculated by dividing the odds of a liver injury event reported for the drug of interest by the odds of a liver injury event reported for the comparison drugs. Additionally, we performed stratified analyses by age (< 65 years vs. > = 65 years), sex (female vs. male), and country where the reports were created (within the United States vs. outside of the United States). We defined a signal of increased risk using a ROR ≥ 2 with a chi-squared test statistic ≥ 4 [8]. Data analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, NC).
Results
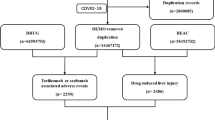
A total of 19,782 patients with COVID-19 (845 for lopinavir-ritonavir and 18,937 for all other drugs combined) were identified within FAERS from 2020 Q1 to 2021 Q1. Of those, 3425 were reported to have a drug-induced liver injury. The flowchart of the identification of drug-induced liver injury in patients with COVID-19 was presented in Fig. 1. The basic characteristics of COVID-19 cases were reported in Table 1.
For lopinavir-ritonavir, 313/845 (37.0%) of reported adverse events were for drug-induced liver injury, compared to 3,112/18,937 (16.4%) for all other drugs combined. The ROR for drug-induced liver injury was 2.99 (95% CI, 2.59–3.46) when comparing lopinavir-ritonavir with all other drugs (Table 2). When lopinavir-ritonavir was compared with hydroxychloroquine/chloroquine only and remdesivir, the ROR for drug-induced liver injury was 3.16 (95% CI, 2.68–3.73) and 5.39 (95% CI, 4.63–6.26), respectively (Table 2). For severe drug-induced liver injury, RORs for lopinavir-ritonavir provided evidence of an association compared with all other drugs (ROR, 3.98; 95% CI, 3.15–5.05), compared with hydroxychloroquine/chloroquine (ROR, 5.33; 95% CI, 4.09–6.94), and compared with remdesivir (ROR, 3.85; 95% CI, 3.03–4.89) (Table 2).
When conducting further analyses stratified by age (< 65 years vs. > = 65 years), sex (female vs. male), and country where the reports were created (within the United States outside of the United States), we also detected a signal of increased risk of both drug-induced liver injury and severe drug-induced liver injury associated with lopinavir-ritonavir in patients with COVID-19 (Table 3).
Discussion
In this disproportionality analysis of FAERS data, we detected signals of increased risks of both drug-induced liver injury and severe drug-induced liver injury associated with lopinavir-ritonavir versus all other drugs, hydroxychloroquine/chloroquine, and remdesivir in patients with COVID-19. The results from the further stratified analyses supported our findings.
Lopinavir-ritonavir has been reported to be associated with moderate-to-severe elevations in serum aminotransferase levels in prior studies [3, 9], which supported our finding that lopinavir-ritonavir might be associated with an increased risk of drug-induced liver injury. Additionally, our results were consistent with the evidence from recent studies [10, 11]. One retrospective case series in China showed that a higher proportion of patients with abnormal liver function had taken lopinavir-ritonavir after admission than those with normal liver function (57.8% vs. 31.3%) [11]. Another case series of 417 patients with COVID-19 also found that the use of lopinavir/ritonavir was associated with increased odds of liver injury (OR from 4.44 to 5.03, both p < 0.01).10 The mechanism of lopinavir-ritonavir induced hepatotoxicity may be due to its metabolism by the cytochrome P450 (CYP) system (primarily CYP3A4) in the liver[12]. Physicians should be aware of the potential risks, including liver injury especially when combining lopinavir-ritonavir with the drugs metabolized by CYP450 enzyme (e.g., chloroquine and hydroxychloroquine) [13].
Moreover, it should be noted that the role of lopinavir-ritonavir in the treatment and preventing of COVID-19 has changed over time. In the early pandemic, lopinavir-ritonavir was considered to be a potential treatment for COVID-19, but then the following trials found that the use of lopinavir-ritonavir did not significantly enhance clinical improvement in adults hospitalized with severe COVID-19 [5, 14]. Accordingly, the guidelines from WHO [15] and Infections Disease Society of America (IDSA) [16] recommended against treating with lopinavir-ritonavir in hospitalized patients with COVID-19. Hydroxychloroquine/chloroquine, another repurposed drug for treating COVID-19, has also been withdrawn the emergency use authorization to treat hospitalized patients with COVID-19 by the US FDA [17], and that use of the agent is not supported by WHO [15].
This study has limitations. Spontaneous events reporting is subject to reporting bias and the lack of denominators to estimate risks of adverse events. Given the limited information available in the FAERS database, we cannot control the confounders in our analysis, which include comorbidities, concomitant medications, or severity of COVID-19. Although disproportionality analysis is a useful tool for signal detection, causality regarding the association between lopinavir-ritonavir and drug-induced liver injury cannot be established by this study, and further research is needed to supplement our findings.
Conclusion
In conclusion, we observed a disproportional signal for drug-induced liver injury associated with lopinavir-ritonavir in patients with COVID-19, indicating that patients treated with lopinavir-ritonavir may be at increased risk of liver injury. Given little evidence of the benefit of the use of lopinavir-ritonavir in patients with COVID-19, WHO has recommended against the use of lopinavir-ritonavir in hospitalized patients with COVID-19. Further studies that evaluate the role of lopinavir-ritonavir in patients with COVID-19 are not warranted and need prior careful ethical consideration. The risks of the over-zealous use of repurposed drugs to treat patients with COVID-19 should be carefully considered.
References
Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506.
Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–30.
Olry A, Meunier L, Delire B, Larrey D, Horsmans Y, Le Louet H. Drug-Induced Liver Injury and COVID-19 Infection: The Rules Remain the Same. Drug Saf. 2020;43(7):615–7
Osborne V, Davies M, Lane S, Evans A, Denyer J, Dhanda S, Roy D, Shakir S. Lopinavir-ritonavir in the treatment of COVID-19: a dynamic systematic benefit-risk assessment. Drug Saf. 2020;43(8):809–21.
Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, Ruan L, Song B, Cai Y, Wei M, Li X, Xia J, Chen N, Xiang J, Yu T, Bai T, Xie X, Zhang L, Li C, Yuan Y, Chen H, Li H, Huang H, Tu S, Gong F, Liu Y, Wei Y, Dong C, Zhou F, Gu X, Xu J, Liu Z, Zhang Y, Li H, Shang L, Wang K, Li K, Zhou X, Dong X, Qu Z, Lu S, Hu X, Ruan S, Luo S, Wu J, Peng L, Cheng F, Pan L, Zou J, Jia C, Wang J, Liu X, Wang S, Wu X, Ge Q, He J, Zhan H, Qiu F, Guo L, Huang C, Jaki T, Hayden FG, Horby PW, Zhang D, Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382(19):1787–99
Gahr M, Zeiss R, Lang D, Connemann BJ, Hiemke C, Schonfeldt-Lecuona C. Drug-induced liver injury associated with antidepressive psychopharmacotherapy: an explorative assessment based on quantitative signal detection using different MedDRA terms. J Clin Pharmacol. 2016;56(6):769–78.
Rothman KJ, Lanes S, Sacks ST. The reporting odds ratio and its advantages over the proportional reporting ratio. Pharmacoepidemiol Drug Saf. 2004;13(8):519–23.
Almenoff JS, Pattishall EN, Gibbs TG, DuMouchel W, Evans SJ, Yuen N. Novel statistical tools for monitoring the safety of marketed drugs. Clin Pharmacol Ther. 2007;82(2):157–66
Sherman KE, Shire NJ, Cernohous P, Rouster SD, Omachi JH, Brun S, Da Silva B. Liver injury and changes in hepatitis C Virus (HCV) RNA load associated with protease inhibitor-based antiretroviral therapy for treatment-naive HCV-HIV-coinfected patients: lopinavir-ritonavir versus nelfinavir. Clin Infect Dis. 2005;41(8):1186–95.
Cai Q, Huang D, Yu H, Zhu Z, Xia Z, Su Y, Li Z, Zhou G, Gou J, Qu J, Sun Y, Liu Y, He Q, Chen J, Liu L, Xu L. COVID-19: Abnormal liver function tests. J Hepatol. 2020;73(3):566–74.
Fan Z, Chen L, Li J, Cheng X, Yang J, Tian C, Zhang Y, Huang S, Liu Z, Cheng J. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18(7):1561–6.
Nunez M. Hepatotoxicity of antiretrovirals: incidence, mechanisms and management. J Hepatol. 2006;44(1 Suppl):S132–9.
Schrezenmeier E, Dorner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16(3):155–66.
Recovery Collaborative Group. Lopinavir-ritonavir in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;396(10259):1345–52.
Rochwerg B, Agarwal A, Siemieniuk RA, Agoritsas T, Lamontagne F, Askie L, Lytvyn L, Leo YS, Macdonald H, Zeng L, Amin W, Burhan E, Bausch FJ, Calfee CS, Cecconi M, Chanda D, Du B, Geduld H, Gee P, Harley N, Hashimi M, Hunt B, Kabra SK, Kanda S, Kawano-Dourado L, Kim YJ, Kissoon N, Kwizera A, Mahaka I, Manai H, Mino G, Nsutebu E, Preller J, Pshenichnaya N, Qadir N, Sabzwari S, Sarin R, Shankar-Hari M, Sharland M, Shen Y, Ranganathan SS, Souza JP, Stegemann M, De Sutter A, Ugarte S, Venkatapuram S, Dat VQ, Vuyiseka D, Wijewickrama A, Maguire B, Zeraatkar D, Bartoszko JJ, Ge L, Brignardello-Petersen R, Owen A, Guyatt G, Diaz J, Jacobs M, Vandvik PO. A living WHO guideline on drugs for covid-19. BMJ. 2020;370m3379.
Infections disease society of America. IDSA guidelines on the treatment and management of patients with COVID-19. 2021 June 25,2021 [cited 2021 July 8]; Available from: https://www.idsociety.org/practice-guideline/covid-19-guideline-treatment-and-management/.
US Food and Drug Administration. Coronavirus (COVID-19) Update: FDA revokes emergency use authorization for chloroquine and hydroxychloroquine. 2020 06/15 [cited 2021 July 9]; Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-revokes-emergency-use-authorization-chloroquine-and.
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
HT is a consultant for Evidpro, LLC. The other authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tang, H., Zhou, L., Li, X. et al. Drug-induced liver injury associated with lopinavir-ritonavir in patients with COVID-19: a disproportionality analysis of U.S. food and drug administration adverse event reporting system (FAERS) data. Int J Clin Pharm 43, 1116–1122 (2021). https://doi.org/10.1007/s11096-021-01311-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-021-01311-5