Abstract
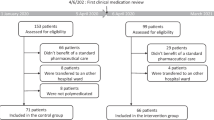
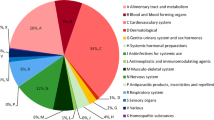
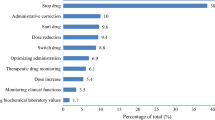
Background Clinical pharmacists’ involvement has improved patients’ care, by suggesting therapeutic optimizations. However, budget restrictions require a prioritization of these activities to focus resources on patients more at risk of medication errors. Objective The aim of our study was to identify variables influencing the formulation of pharmaceutical to improve medication review efficiency. Setting This study was conducted in medical wards of a 643-acute beds hospital in Paris, France. Methods All hospital medical prescriptions of all patients admitted within four medical wards (cardiology, rheumatology, neurology, vascular medicine) were analyzed. The study was conducted in each ward for 2 weeks, during 4 weeks. For each patient, variables prospectively collected were: age, gender, weight, emergency admission, number of high-alert medications and of total drugs prescribed, care unit, serum creatinine. Number of pharmaceutical interventions (PIs) and their type were reported. Main outcome measures Variables influencing the number of pharmaceutical interventions during medication review were identified using simple and multiple linear regressions. Results A total of 2328 drug prescriptions (303 patients, mean age 70.6 years-old) were analyzed. Mean number of hospital drug prescriptions was 7.9. A total of 318 PIs were formulated. Most frequent PIs were drug omission (n = 88, 27.7%), overdosing (n = 69, 21.7%), and underdosing (n = 51, 16.0%). Among variables studied, age, serum creatinine level, number of high-alert medications prescribed and total number of drugs prescribed were significantly associated with the formulation of pharmaceutical interventions (adjusted R2 = 0.34). Conclusions This study identified variables (age, serum creatinine level, number of high-alert medication, number of prescribed drugs) that may help institutions/pharmacists target their reviews towards patients most likely to require pharmacist interventions.
Similar content being viewed by others
References
European Medicines Agency - Pharmacovigilance - Medication errors. http://www.ema.europa.eu/ema/index.jsp?curl=pages/special_topics/general/general_content_000570.jsp. Accessed 31 Jan 2018.
Kuo GM, Touchette DR, Marinac JS, For the American College of Clinical Pharmacy Practice-Based Research Network Collaborative. Drug errors and related interventions reported by United States clinical pharmacists: the American College of clinical pharmacy practice-based research network medication error detection, amelioration and prevention study. Pharmacother J Hum Pharmacol Drug Ther. 2013;33:253–65.
Bates DW, Cullen DJ, Laird N, Petersen LA, Small SD, Servi D, Laffel G, Sweitzer BJ, Shea BF, Hallisey R, et al. Incidence of adverse drug events and potential adverse drug events. Implications for prevention. ADE Prevention Study Group. JAMA. 1995;274(1):29–34.
Santell JP, Hicks RW, McMeekin J, Cousins DD. Medication errors: experience of the United States Pharmacopeia (USP) MEDMARX reporting system. J Clin Pharmacol. 2003;43:760–7.
Brazinha I, Fernandez-Llimos F. Barriers to the implementation of advanced clinical pharmacy services at Portuguese hospitals. Int J Clin Pharm. 2014;36(5):1031–8.
Minard LV, Deal H, Harrison ME, Toombs K, Neville H, Meade A. Pharmacists’ perceptions of the barriers and facilitators to the implementation of clinical pharmacy key performance indicators. PLoS One. 2016;11(4):e0152903.
Pfister B, Jonsson J, Gustafsson M. Drug-related problems and medication reviews among old people with dementia. BMC Pharmacol Toxicol. 2017;18:52.
Légifrance. Arrêté du 6 avril 2011 relatif au management de la qualité de la prise en charge médicamenteuse et aux médicaments dans les établissements de santé. 2011. https://www.legifrance.gouv.fr/eli/arrete/2011/4/6/ETSH1109848A/jo. Accessed 31 Jan 2018.
ISMP. High-Alert Medications. 2014. http://www.ismp.org/Tools/institutionalhighAlert.asp. Accessed 31 Jan 2018.
NHS High Risk Drugs List. 2011. http://www.sssft.nhs.uk/services/pharmacy/information-for-professionals/high-risk-drugs-list. Accessed 31 Jan 2018.
Clinical Pharmacy French Society (Société Française de Pharmacie Clinique). ActIP® software. http://www.actip.sfpc.eu/actip/index. Accessed 13 Apr 2018.
Vande Griend JP, Saseen JJ, Bislip D, Emsermann C, Conry C, Pace WD. Prioritization of patients for comprehensive medication review by a clinical pharmacist in family medicine. J Am Board Fam Med. 2015;28(3):418–24.
Stordeur F, Khouri T, Lehrer J, Beaussier H, Bezie Y, Phan Thi TT. Prescriptions analysis: how can we target our work? Eur J Hosp Pharm. 2017;24:A101–2.
Jarré C, Bouchet J, Hellot-Guersin M, Ferry JM, Leromain AS, Derharoutunian C, et al. ACESO : une requête pour la sélection des ordonnances à risque. 2016. http://docplayer.fr/43107190-Aceso-une-requete-pour-la-selection-des-ordonnances-a-risque.html. Accessed 31 Jan 2018.
Vande Griend JP, Saseen JJ, Bislip D, Emsermann C, Conry C, Pace WD. Prioritization of patients for comprehensive medication review by a clinical pharmacist in family medicine. J Am Board Fam Med. 2015;28(3):418–24.
Mandal K, Fraser S. The incidence of prescribing errors in an eye hospital. BMC Ophthalmol. 2005;5(1):4.
Orion Health. Introduction to Machine Learning. 2016. http://web.orionhealth.com/rs/981-HEV-035/images/Introduction_to_Machine_Learning.pdf. Accessed 13 Apr 2018.
Motwani M, Dey D, Berman DS, Germano G, Achenbach S, Al-Mallah MH, et al. Machine learning for prediction of all-cause mortality in patients with suspected coronary artery disease: a 5-year multicentre prospective registry analysis. Eur Heart J. 2017;38(7):500–7.
Acknowledgements
Authors thank Nicolas Greliche, statistician, for his advices, revision of the manuscript and his participation in statistical analyses of this manuscript.
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Cornuault, L., Mouchel, V., Phan Thi, TT. et al. Identification of variables influencing pharmaceutical interventions to improve medication review efficiency. Int J Clin Pharm 40, 1175–1179 (2018). https://doi.org/10.1007/s11096-018-0668-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-018-0668-y