Abstract
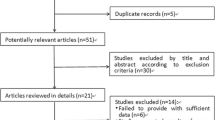
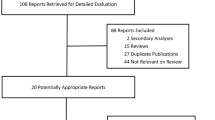
Background Multiple studies have compared the efficacy of entecavir with lamivudine in preventing hepatitis B virus (HBV) reactivation among HBV-carrying lymphoma patients with chemotherapy treatment. However, the results were slightly varied. Aim of the review to combine the findings of independent studies assessing the clinical efficacy of the two drugs using a systematic review and meta-analysis. Methods PubMed, China National Knowledge Infrastructure (CNKI), Chinese Biomedical Literature Database (CBM), Chongqing VIP and WanFang Data were retrieved. Two independent reviewers evaluated the study eligibility and extracted eight studies, with 770 patients in total. The meta-analysis was conducted using RevMan 5.3 and STATA software. Results HBV-carrying lymphoma patients receiving lamivudine during chemotherapy had a statistically significantly higher odds of HBV reactivation compared to those receiving entecavir (OR 5.0, 95 % CI 2.85–8.78, P < 0.001). The odds of hepatitis, HBV-Reactivation caused hepatitis and chemotherapy disruption was statistically significantly elevated in the patient group receiving lamivudine compared to the entecavir group (OR 4.12, 95 % CI 1.70–9.98, P = 0.002; OR 11.44, 95 % CI 2.70–48.52, P < 0.001; OR 6.71, 95 % CI 2.34–19.26, P < 0.001, respectively). Furthermore, the HBV reactivation rate in Ann Arbor stages I - II patient group was statistically significantly lower than the one in Ann Arbor stages III–IV group, with an overall pooled value of 0.37 (95 % CI 0.17–0.82, P = 0.01). Conclusion The metaanalysis result suggested that among HBV-carrying lymphoma patients undergoing chemotherapy, entecavir is more effective than lamivudine in preventing HBV reactivation.




Similar content being viewed by others
References
Kim SJ, Hsu C, Song YQ, Tay K, Hong XN, Cao J, et al. Hepatitis B virus reactivation in B-cell lymphoma patients treated with rituximab: analysis from the Asia Lymphoma Study Group. Eur J Cancer. 2013;49(16):3486–96.
Li H, Zhang HM, Chen LF, Chen YQ, Chen L, Ren H, et al. Prophylactic lamivudine to improve the outcome of HBsAg-positive lymphoma patients during chemotherapy: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. 2015;39(1):80–92.
Park SC, Jeong SH, Kim J, Han CJ, Kim YC, Choi KS, et al. High prevalence of hepatitis B virus infection in patients with B-cell non-Hodgkin’s lymphoma in Korea. J Med Virol. 2008;80(6):960–6.
Yeo W, Chan PK, Zhong S, Ho WM, Steinberg JL, Tam JS, et al. Frequency of hepatitis B virus reactivation in cancer patients undergoing cytotoxic chemotherapy: a prospective study of 626 patients with identification of risk factors. J Med Virol. 2000;62(3):299–307.
Yeo W, Zee B, Zhong S, Chan PK, Wong WL, Ho WM, et al. Comprehensive analysis of risk factors associating with hepatitis B virus (HBV) reactivation in cancer patients undergoing cytotoxic chemotherapy. Br J Cancer. 2004;90(7):1306–11.
Hwang J, Barbo A, Perrillo R. Hepatitis B reactivation during cancer chemotherapy: an international survey of the membership of the American Association for the Study of Liver Diseases. J Viral Hepat. 2015;22(3):346–52.
Lau GK, Yiu HH, Fong DY, Cheng HC, Au WY, Lai LS, et al. Early is superior to deferred preemptive lamivudine therapy for hepatitis B patients undergoing chemotherapy. Gastroenterology. 2003;125(6):1742–9.
Dong HJ, Ni LN, Sheng GF, Song HL, Xu JZ, Ling Y. Risk of hepatitis B virus (HBV) reactivation in non-Hodgkin lymphoma patients receiving rituximab-chemotherapy: a meta-analysis. J Clin Virol. 2013;57(3):209–14.
Hui CK, Cheung WW, Zhang HY, Au WY, Yueng YH, Leung AY, et al. Kinetics and risk of de novo hepatitis B infection in HBsAg–negative patients undergoing cytotoxic chemotherapy. Gastroenterology. 2006;131(1):59–68.
Loomba R, Rowley A, Wesley R, Liang TJ, Hoofnagle JH, Pucino F, et al. Systematic review: the effect of preventive lamivudine on hepatitis B reactivation during chemotherapy. Ann Intern Med. 2008;148(7):519–28.
Jarvis B, Faulds D. Lamivudine. A review of its therapeutic potential in chronic hepatitis B. Drugs. 1999;58(1):101–41.
Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, et al. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124(1):105–17.
Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339(2):61–8.
Law JK, Ali JA, Harrigan PR, Sherlock CH, Savage KJ, Yoshida EM. Fatal postlymphoma chemotherapy hepatitis B reactivation secondary to the emergence of a YMDD mutant strain with lamivudine resistance in a noncirrhotic patient. Am J Hematol. 2006;81(12):969–72.
Liaw YF. Management of YMDD mutations during lamivudine therapy in patients with chronic hepatitis B. J Gastroenterol Hepatol. 2002;17(s3):S333–7.
Innaimo SF, Seifer M, Bisacchi GS, Standring DN, Zahler R, Colonno RJ. Identification of BMS-200475 as a potent and selective inhibitor of hepatitis B virus. Antimicrob Agents Chemother. 1997;41(7):1444–8.
Gish RG, Lok AS, Chang TT, de Man RA, Gadano A, Sollano J, et al. Entecavir therapy for up to 96 weeks in patients with HBeAg-positive chronic hepatitis B. Gastroenterology. 2007;133(5):1437–44.
Li HR, Huang JJ, Guo HQ, Zhang X, Xie Y, Zhu HL, et al. Comparison of entecavir and lamivudine in preventing hepatitis B reactivation in lymphoma patients during chemotherapy. J Viral Hepat. 2011;18(12):877–83.
Huang J, Chen XP, Chen XF, Chen WL. Clinical observation of preventing and treating HBV reactivation by lamivudine and entecavirin patients with non-Hodgkin lymphoma. J Prac Med. 2011;27(12):2225–7.
Lok AS, Liang RH, Chiu EK, Wong KL, Chan TK, Todd D. Reactivation of hepatitis B virus replication in patients receiving cytotoxic therapy. Rep Prospect Stud. Gastroenterol. 1991;100(1):182–8.
Huang H, Li X, Zhu J, Ye S, Zhang H, Wang W, et al. Entecavir versus lamivudine for prevention of hepatitis B virus reactivation among patients with untreated diffuse large B-cell lymphoma receiving R-CHOP chemotherapy: a randomized clinical trial. JAMA. 2014;312(23):2521–30.
Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5.
Chen YM, Qian SX, Xie YP, Yang F. Comparison of lamivudine and entecavir in preventing hepatitis B reactivation in B-cell non-Hodgkin’S lymphoma patients during chemotherapy and the risk factors of hepatitis B occurrence. Zhejiang Med J. 2014;36(11):941–4.
Ziakas PD, Karsaliakos P, Mylonakis E. Effect of prophylactic lamivudine for chemotherapy-associated hepatitis B reactivation in lymphoma: a meta-analysis of published clinical trials and a decision tree addressing prolonged prophylaxis and maintenance. Haematologica. 2009;94(7):998–1005.
Alagozlu H, Ozdemir O, Koksal B, Yilmaz A, Coskun M. Prevelance of common YMDD motif mutations in long term treated chronic HBV infections in a Turkish population. Asian Pac J Cancer Prev. 2013;14(9):5489–94.
Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661–2.
Ni CB, Long B. Clinical comparison of preventive and therapeutic effects of lamivudine and entecavir on HBV reactivation among patients with non-Hodgkin lymphoma. J Clin Hepatol. 2014;30(4):363–6.
Tan Y, Wu HF, Zou ML, Pan HC, Hu ZP, Wu JY. Comparison study of entecavir and lamivudine in treating hepatitis B reactivation in Non-Hodgkin lymphoma patients. Jiangxi Med J. 2014;49(9):854-67.
Chang TT, Gish RG, de Man R, Gadano A, Sollano J, Chao YC, et al. A comparison of entecavir and lamivudine for HBeAg-positive chronic hepatitis B. N Engl J Med. 2006;354(10):1001–10.
Lai CL, Shouval D, Lok AS, Chang TT, Cheinquer H, Goodman Z, et al. Entecavir versus lamivudine for patients with HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 2006;354(10):1011–20.
Rivkin A. Entecavir: a new nucleoside analogue for the treatment of chronic hepatitis B. Drugs Today. 2007;43(4):201–20.
Tenney DJ, Rose RE, Baldick CJ, Pokornowski KA, Eggers BJ, Fang J, et al. Long-term monitoring shows hepatitis B virus resistance to entecavir in nucleoside-naive patients is rare through 5 years of therapy. Hepatology. 2009;49(5):1503–14.
Sherman M, Yurdaydin C, Sollano J, Silva M, Liaw YF, Cianciara J, et al. Entecavir for treatment of lamivudine-refractory, HBeAg-positive chronic hepatitis B. Gastroenterology. 2006;130(7):2039–49.
Sherman M, Yurdaydin C, Simsek H, Silva M, Liaw YF, Rustgi VK, et al. Entecavir therapy for lamivudine-refractory chronic hepatitis B: improved virologic, biochemical, and serology outcomes through 96 weeks. Hepatology. 2008;48(1):99–108.
Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–101.
Acknowledgments
We thank Pan ML and Luis AP from the University of Texas Health Science Center for its linguistic assistance during the preparation of this manuscript and the reviewers for their intellectual support.
Funding
No special funding was received for this review.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
There is no conflict of interests.
Additional information
Sisi Yuand and Huaichao Luo have contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
11096_2016_358_MOESM1_ESM.tif
Supplementary Fig. 1 Funnel plot analysis for publication bias (A) Funnel plot for HBV reactivation rate (the lamivudine prophylaxis group vs. entecavir prophylaxis group) with all 8 studies, P for publication bias is 0.108; (B) Funnel plot for the incidence of hepatitis (the lamivudine prophylaxis group vs. entecavir prophylaxis group), P for publication bias is 1.00; (C) Funnel plot for the incidence of hepatitis due to HBV reactivation (the lamivudine prophylaxis group vs. entecavir prophylaxis group), P for publication bias is 0.296 (TIFF 280 kb)
11096_2016_358_MOESM2_ESM.tif
Supplementary Fig. 2 Funnel plot analysis for publication bias (A) Funnel plot for the incidence of chemotherapy disruption (the lamivudine prophylaxis group vs. entecavir prophylaxis group), P for publication bias is 0.296; (B) Funnel plot for HBV reactivation rate (Ann Arbor stage I–II group vs. Ann Arbor stage III–IV group), P for publication bias is 0.734 (TIFF 20526 kb)
Rights and permissions
About this article
Cite this article
Yu, S., Luo, H., Pan, M. et al. Comparison of entecavir and lamivudine in preventing HBV reactivation in lymphoma patients undergoing chemotherapy: a meta-analysis. Int J Clin Pharm 38, 1035–1043 (2016). https://doi.org/10.1007/s11096-016-0358-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-016-0358-6