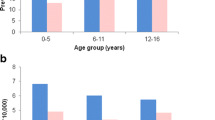
Abstract
Background Epileptic seizures are neurological disorders common in children; 4 to 10 % of under 16-year olds have suffered at least one seizure. Antiepileptic drugs represent the basis of treatment for the majority of patients, but many of the drugs prescribed to pediatrics are used unlicensed and off-label. Objective To analyze the prevalence of use of unlicensed and off-label antiepileptic drugs, by the pediatric population, according to the Food and Drug Administration and the Brazilian National Health Surveillance Agency. Setting General Hospital of the Faculty of Medicine at Ribeirão Preto, Brazil. Methods A cross-sectional, retrospective and observational study was carried out. The daily prescriptions of children up to 12 years of age were collected for the analysis of antiepileptic drug use. Data of the registration number, sex, age, reason hospitalized, unit where hospitalized, drug prescribed, dosage, route and administration frequency were collected. Main outcome measure Antiepileptic drugs prescribed for children were classified as unlicensed and off-label according to the term of the product’s license registered in the Food and Drug Administration and the Brazilian National Health Surveillance Agency. Results Of the 6,637 pediatric patients identified during the study period, 583 (9.0 %) received at least one antiepileptic drug. The most used antiepileptic drugs were phenobarbital, phenytoin, carbamazepine, valproic acid and clonazepam. As expected, the number of pharmaceutical dosage form classified as unlicensed or off-label was high in both agencies, but distinct between the two. The number of patients (n = 287) using unlicensed drugs was similar in the two agencies, but the use of off-label drugs was higher according to the analysis carried out by the North American agency (40.5 %). Conclusions Old-generation antiepileptic drugs are widely prescribed to children. The results found for the use of off-label drugs demonstrate the absence of uniformity in action between the agencies and a lack of integration between the studies carried out. Although legislation on the licensing of drugs aims to protect the patients from drugs that have not been scientifically evaluated, the scarcity of data about the safety of the therapeutic resources obliges the doctors to prescribe unlicensed and off-label antiepileptic drugs to the pediatric population.


Similar content being viewed by others
References
Hoppu K. Paediatric clinical pharmacology—at the beginning of a new era. Eur J Clin Pharmacol. 2008;64:201–5.
World Health Organization. Promoting safety of medicines for children. Geneva: WHO; 2007. ISBN 978-92-4-156343-7.
Wilson JT. An update on the therapeutic orphan. Pediatrics. 1999;104:585–90.
Choonara I, Conroy S. Unlicensed and off-label drug use in children—implications for safety. Drug Saf. 2002;25:1–5.
Conroy S. Unlicensed and off-label drug use—issues and recommendations. Paediatr Drugs. 2002;4:353–9.
Hanssens Y, Deleu D, Al Balushi K, Al Hashar A, Al-Zakwani I. Drug utilization pattern of anti-epileptic drugs: a pharmacoepidemiologic study in Oman. J Clin Pharm Ther. 2002;27:357–64.
Koshy S. Role of pharmacists in the management of patients with epilepsy. Int J Pharm Pract. 2012;20:65–8.
Kimland E, Nydert P, Odlind V, Böttiger Y, Lindemalm S. Pediatric drug use with focus on off-label prescriptions at Swedish hospital—a nationwide study. Acta Paediatr. 2012;101:1–7.
Morales-Carpi C, Estañ L, Rubio E, Lurbe E, Morales-Olivas FJ. Drug utilization and off-label drug use among Spanish emergency room pediatric patients. Eur J Clin Pharmacol. 2010;66:315–20.
Clarkson A, Choonara I. Surveillance for fatal suspected adverse drug reaction in the UK. Arch Dis Child. 2002;87:462–7.
Friedman DOMJ, Sharieff MDGQ. Seizures in children. Pediatr Clin N Am. 2006;53:257–77.
Chen LC, Chen YF, Yang LL, Chou MH, Lin MF. Drug utilization pattern of antiepileptic drugs and traditional Chinese medicines in a general hospital in Taiwan—a pharmaco-epidemiologic study. J Clin Pharm Ther. 2000;25:125–9.
Food and Drug Administration. Guidance for industry: E11 clinical investigation of medicinal products in the pediatric population. Rockille, Md. 2000. Available: http://www.fda.gov/downloads/RegulatoryInformation/Guidances/ucm129477.pdf. Accessed 30 Aug 2012.
Turner S, Longworth A, Nunn AJ, Choonara I. Unlicensed and off label drug use in paediatric wards: prospective study. BMJ. 1998;316:343–5.
Abasolo-Osinaga E, Abecia-Inchaurregui LC, Etxeandia-Ikobaltzeta I, Burgos-Alonso N, Pozo JG. Estudio farmacoepidemiológico del consumo de fármacos antiepilépticos (1992–2004). Rev Neurol. 2008;46:449–53.
Chen AH. Update on pediatric epilepsy. Adv Pediatr. 2011;58:259–76.
Hasan SS, Bahari MB, Bazar ZU, Ganesan V. Antiepileptic drug utilization and seizure outcome among pediatric patients in a Malaysian public hospital. Singap Med J. 2010;51:21–7.
Deckers CLP, Hekstert YA, Keyser A, Meinardi H, Reiner WO. Pharmacotherapy of epilepsy: state of the art and developments. J Clin Pharm Ther. 1997;22:309–22.
Ministério da Saúde (Brazil). PORTARIA SAS/MS No 492, de 23 de setembro de 2010. Protocolo Clínico e Diretrizes Terapêuticas—Epilepsia. [Brazilian Therapeutic Guideline—Epilepsy]. Available: http://portal.saude.gov.br/portal/arquivos/pdf/pcdt_epilepsia_.pdf. Accessed 05 April 2012.
Glauser T, Ben-Menachem E, Borgeous B, Cnaan A, Chadwick D, Guerreiro C, et al. ILAE treatment guidelines: evidence-based analysis of antiepileptic drug efficacy and effectiveness as initial monotherapy for epileptic seizures and syndromes. Epilepsia. 2006;47:1094–120.
Abend NS, Gutierrez-Colina AM, Dlugos DJ. Medical treatment of pediatric status epileptics. Semin Pediatr Neurol. 2010;17:169–75.
Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342(5):314–9.
Nicholas JM, Ridsdale L, Richardson MP, Ashworth M, Gulliford MC. Trends in antiepileptic drug utilisation in UK primary care 1993–2008: cohort study using the general practice research database. Seizure. 2012;21(6):466–70.
Perucca E. Clinically relevant drug interaction with antiepileptic drugs. Br J Clin Pharmacol. 2005;61:246–55.
Johannessen SI, Landmark CJ. Antiepileptic drug interactions—principles and clinical implications. Curr Neuropharmacol. 2010;8:254–67.
Yi ZM, Zhai SD, Huang S, Wang TS, Liu F. Off-label prescriptions for adult neurological patients: a pilot survey in China. Int J Clin Pharm. 2012;34(1):81–7.
Conroy S, Choonara I, Impicciatore P, Mohn A, Arnell H, Rane A, et al. Survey of unlicensed and off-label drug use in paediatric wards in European countries. BMJ. 2000;7227(320):79–82.
Shah SS, Goodman D, Feur P, Sharma V, Fargason C Jr, Hyman D, et al. Off-label drug use in hospitalized children. Arch Pediatr Adolesc Med. 2007;161:282–90.
Dell’Aera M, Gasbarro AR, Padovano M, Laforgia N, Capodiferro D, Solarino B, et al. Unlicensed and off-label use of medicines at a neonatology clinic in Italy. Pharm World Sci. 2007;29(4):361–7.
Di Paolo ER, Stoetter H, Cotting J, Frey P, Gehri M, Beck-Popovic M, et al. Unlicensed and off-label drug use in a Swiss pediatric university hospital. Swiss Med Wkly. 2006;136:218–22.
‘t Jong GW, van der Linden PD, Bakker EM, van der Lely N, Eland IA, Stricker BH, van den Anker JN. Unlicensed and off-label use in a pediatric ward of a general hospital in the Netherlands. Eur J Clin Pharmacol. 2002;58:293–7.
Schirm E, Tobi H, Berg LTWJ. Risk factor for unlicensed and off-label drug use in children outside the hospital. Pediatrics. 2003;111:291–5.
Agência Nacional de Vigilância Sanitária (Brazil). Medicamentos. [Brazilian National Health Surveillance Agency. Drugs]. Available: http://portal.anvisa.gov.br/wps/content/Anvisa+Portal/Anvisa/Inicio/Medicamentos. Accessed 20 Feb 2011.
Acknowledgments
The authors would like to thank the directors of the General Hospital of the Faculty of Medicine at Ribeirão Preto of the University of São Paulo (HCFMRP-USP) for their assistance in data collection.
Funding
The study was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Borges, A.P.d.S., Campos, M.S.d.A. & Pereira, L.R.L. Evaluation of unlicensed and off-label antiepileptic drugs prescribed to children: Brazilian Regulatory Agency versus FDA. Int J Clin Pharm 35, 425–431 (2013). https://doi.org/10.1007/s11096-013-9755-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11096-013-9755-2