Abstract
Purpose
Etodolac (ETD) is one of the non-steroidal anti-inflammatory drugs which has low aqueous solubility issues. The objective of this study was to develop ETD nanosuspensions to improve its poor aqueous solubility properties while investigating formulation and process parameters of wet media milling method via design of experiment (DoE) approach.
Methods
The critical formulation parameters (CFP) were selected as ETD amount, stabilizer type and ratio as well as critical process parameters (CPP) which were bead size, milling time and milling speed. The two-factorial-23 and The Box-Benkhen Designs were generated to evaluate CFP and CPP, respectively. Particle size (PS), polydispersity index (PDI) and zeta potential (ZP) were analyzed as dependent variables. Characterization, physical stability and solubility studies were performed.
Results
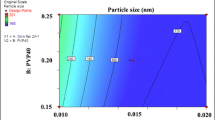
Optimum nanosuspensions stabilized by PVP K30 and Poloxamer 188 showed 188.5 ± 1.6 and 279.3 ± 6.1 nm of PS, 0.161 ± 0.049 and 0.345 ± 0.007 PDI, 14.8 ± 0.3 and 16.5 ± 0.4 mV of ZP values, respectively. The thermal properties of ETD did not change after milling and lyophilization process regarding to DSC analysis. Also, the crystalline state of ETD was preserved. The morphology of particle was smooth and spherical on SEM. The dry-nanosuspensions stayed physically stable for six months at room temperature. The solubility of nanosuspensions increased up to 13.0-fold in comparison with micronized ETD.
Conclusions
In conclusion, it is found that the poor solubility issue of ETD can be solved by nanosuspension. DoE approach provided benefits such as reducing number of experiments, saving time and improving final product quality by using wet media milling.














Similar content being viewed by others
Abbreviations
- ETD:
-
Etodolac
- NS:
-
Nanosuspension
- CFP:
-
Critical Formulation Parameters
- CPP:
-
Critical Process Parameter
- PS:
-
Particle Size
- PDI:
-
Polydispersity Index
- ZP:
-
Zeta Potential
- Conc.:
-
Concentration
- PVP:
-
Polyvinylpyrrolidone
- BBD:
-
Box-Benkhen Design
- DoE:
-
Design of Experiment
References
Bitterlich A, Laabs C, Krautstrunk I, Dengler M, Juhnke M, Grandeury A, et al. Process parameter dependent growth phenomena of naproxen nanosuspension manufactured by wet media milling. Eur J Pharm Biopharm [Internet]. Elsevier B.V.; 2015;92:171–9. Available from: https://doi.org/10.1016/j.ejpb.2015.02.031
Hatahet T, Morille M, Hommoss A, Dorandeu C, Müller RH, Bégu S. Dermal quercetin smartCrystals®: Formulation development, antioxidant activity and cellular safety. Eur J Pharm Biopharm [Internet]. Elsevier B.V.; 2016;102:51–63. Available from: https://doi.org/10.1016/j.ejpb.2016.03.004
Yao J, Cui B, Zhao X, Wang Y, Zeng Z, Sun C, et al. Preparation, characterization, and evaluation of azoxystrobin nanosuspension produced by wet media milling. Appl Nanosci [internet]. Springer International Publishing; 2018;8:297–307. Available from. 2018. https://doi.org/10.1007/s13204-018-0745-5.
Mishra PR. Shaal L Al, Müller RH, Keck CM. Production and characterization of Hesperetin nanosuspensions for dermal delivery. Int J Pharm. 2009;371:182–9.
De Smet L, Saerens L, De Beer T, Carleer R, Adriaensens P, Van Bocxlaer J, et al. Formulation of itraconazole nanococrystals and evaluation of their bioavailability in dogs. Eur J Pharm Biopharm [Internet]. Elsevier B.V.; 2014;87:107–13. Available from: https://doi.org/10.1016/j.ejpb.2013.12.016
Zuo W, Qu W, Li N, Yu R, Hou Y, Liu Y, et al. Fabrication of multicomponent amorphous bufadienolides nanosuspension with wet milling improves dissolution and stability. Artif Cells, Nanomedicine Biotechnol. 2018;46:1513–22.
Rabinow BE. Nanosuspensions in drug delivery. Nat Rev Drug Discov. 2004;3:785–96.
Müller RH, Gohla S, Keck CM. State of the art of nanocrystals - special features, production, nanotoxicology aspects and intracellular delivery. Eur J Pharm Biopharm. 2011;78:1–9.
Stahr PL, Keck CM. Preservation of rutin nanosuspensions without the use of preservatives. Beilstein J Nanotechnol. 2019;10:1902–13.
Wu W, Nancollas GH. A new understanding of the relationship between solubility and particle size. J Solut Chem. 1998;27:521–31.
Huang S, Zhang Q, Li H, Sun Y, Cheng G, Zou M, et al. Increased bioavailability of efonidipine hydrochloride nanosuspensions by the wet-milling method. Eur J Pharm Biopharm [internet]. Elsevier; 2018;130:108–14. Available from. 2018. https://doi.org/10.1016/j.ejpb.2018.06.022.
Ely DR, Edwin García R, Thommes M. Ostwald-Freundlich diffusion-limited dissolution kinetics of nanoparticles. Powder Technol [Internet]. Elsevier B.V.; 2014;257:120–3. Available from: https://doi.org/10.1016/j.powtec.2014.01.095
Ahmadi Tehrani A, Omranpoor MM, Vatanara A, Seyedabadi M, Ramezani V. Formation of nanosuspensions in bottom-up approach: theories and optimization. DARU, J Pharm Sci. DARU Journal of Pharmaceutical Sciences; 2019;27:451–73.
Müller RH, Keck CM. Twenty years of drug nanocrystals: where are we, and where do we go? Eur J Pharm Biopharm. 2012;80:1–3.
Shegokar R, Müller RH. Nanocrystals: Industrially feasible multifunctional formulation technology for poorly soluble actives. Int J Pharm [Internet]. Elsevier B.V.; 2010;399:129–39. Available from: https://doi.org/10.1016/j.ijpharm.2010.07.044
Peltonen L, Hirvonen J. Pharmaceutical nanocrystals by nanomilling: critical process parameters, particle fracturing and stabilization methods. J Pharm Pharmacol. 2010;62:1569–79.
Raghava Srivalli KM, Mishra B. Drug nanocrystals: a way toward scale-up. Saudi Pharm J [internet]. King Saud University. 2016;24:386–404. Available from:. https://doi.org/10.1016/j.jsps.2014.04.007.
Cui B, Lv Y, Gao F, Wang C, Zeng Z, Wang Y, et al. Improving abamectin bioavailability via nanosuspension constructed by wet milling technique. Pest Manag Sci. 2019;75:2756–64.
Kuroiwa Y, Higashi K, Ueda K, Yamamoto K, Moribe K. Nano-scale and molecular-level understanding of wet-milled indomethacin/poloxamer 407 nanosuspension with TEM, suspended-state NMR, and Raman measurements. Int J Pharm [internet]. Elsevier; 2018;537:30–9. Available from. https://doi.org/10.1016/j.ijpharm.2017.12.028.
Karakucuk A, Teksin ZS, Eroglu H, Celebi N. Evaluation of improved oral bioavailability of ritonavir nanosuspension. Eur J Pharm Sci [internet]. Elsevier; 2019;131:153–8. Available from. https://doi.org/10.1016/j.ejps.2019.02.028.
Keck CM, Müller RH. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenisation. Eur J Pharm Biopharm. 2006;62:3–16.
Patel PJ, Gajera BY, Dave RH. A quality-by-design study to develop Nifedipine nanosuspension: examining the relative impact of formulation variables, wet media milling process parameters and excipient variability on drug product quality attributes. Drug Dev Ind Pharm [internet]. Taylor & Francis; 2018;44:1942–52. Available from. https://doi.org/10.1080/03639045.2018.1503296.
Flach F, Breitung-Faes S, Kwade A. Model based process optimization of nanosuspension preparation via wet stirred media milling. Powder Technol [internet]. Elsevier B.V.; 2018;331:146–54. Available from. https://doi.org/10.1016/j.powtec.2018.03.011.
Karakucuk A, Celebi N, Teksin ZS. Preparation of ritonavir nanosuspensions by microfluidization using polymeric stabilizers: I. a Design of Experiment approach. Eur J Pharm Sci. 2016;95:111–21.
Rangaraj N, Pailla SR, Chowta P, Sampathi S. Fabrication of Ibrutinib Nanosuspension by quality by design approach: intended for enhanced Oral bioavailability and diminished fast fed variability. AAPS PharmSciTech. AAPS PharmSciTech. 2019;20.
Sekharan TR, Muthumari M, Gopal L, Esakiyammal A. Dissolution improvement of Etodolac using Mannitol by solid dispersion method. Sekharan al World J Pharm Pharm Sci. 2014;3:1206–16.
Shaikh A, Praveen C, Pravind L. Central composite Design for Enhancement of Etodolac solubility via inclusion Complexation with Pvp K-30 and Hydroxypropyl Β – Cyclodextrin. Eur J Pharm Med Res. 2017;4:657–63.
Barakat NS. In vitro and in vivo characteristics of a thermogelling rectal delivery system of etodolac. AAPS PharmSciTech. 2009;10:724–31.
Barakat NS. The efficiency of self-emulsification , drug release studies ( dissolution ), in vivo absorption in rat ,, pharmacodynamics about Enhanced oral bioavailability of etodolac by self-emulsifying systems : in-vitro and in-vivo evaluation. Animals. 2010;173–180.
Kumar S, Xu X, Gokhale R, Burgess DJ. Formulation parameters of crystalline nanosuspensions on spray drying processing: A DoE approach. Int J Pharm [Internet]. Elsevier B.V.; 2014;464:34–45. Available from: https://doi.org/10.1016/j.ijpharm.2014.01.013
El Kousy NM. Spectrophotometric and spectrofluorimetric determination of etodolac and aceclofenac. J Pharm Biomed Anal. 1999;20:185–94.
Mora CP, Martínez F. Solubility of naproxen in several organic solvents at different temperatures. Fluid Phase Equilib. 2007;255:70–7.
Baka E, Comer JEA, Takács-Novák K. Study of equilibrium solubility measurement by saturation shake-flask method using hydrochlorothiazide as model compound. J Pharm Biomed Anal. 2008;46:335–41.
Ahuja BK, Jena SK, Paidi SK, Bagri S, Suresh S. Formulation, optimization and in vitro-in vivo evaluation of febuxostat nanosuspension. Int J Pharm [Internet]. Elsevier B.V.; 2015;478:540–52. Available from: https://doi.org/10.1016/j.ijpharm.2014.12.003
Oktay AN, Karakucuk A, Ilbasmis-Tamer S, Celebi N. Dermal flurbiprofen nanosuspensions: optimization with design of experiment approach and in vitro evaluation. Eur J Pharm Sci. 2018;122:254–63.
Gajera BY, Shah DA, Dave RH. Development of an amorphous nanosuspension by sonoprecipitation-formulation and process optimization using design of experiment methodology. Int J pharm [internet]. Elsevier; 2019;559:348–59. Available from. https://doi.org/10.1016/j.ijpharm.2019.01.054.
Wang Y, Zheng Y, Zhang L, Wang Q, Zhang D. Stability of nanosuspensions in drug delivery. J Control Release [Internet]. Elsevier B.V.; 2013;172:1126–41. Available from: https://doi.org/10.1016/j.jconrel.2013.08.006
Liu Q, Guan J, Sun Z, Shen X, Li L, Jin L, et al. Influence of stabilizer type and concentration on the lung deposition and retention of resveratrol nanosuspension-in-microparticles. Int J Pharm [internet]. Elsevier; 2019;569:118562. Available from. https://doi.org/10.1016/j.ijpharm.2019.118562.
Azad M, Afolabi A, Bhakay A, Leonardi J, Davé R, Bilgili E. Enhanced physical stabilization of fenofibrate nanosuspensions via wet co-milling with a superdisintegrant and an adsorbing polymer. Eur J Pharm Biopharm [Internet]. Elsevier B.V.; 2015;94:372–85. Available from: https://doi.org/10.1016/j.ejpb.2015.05.028
Van Eerdenbrugh B, Van den Mooter G, Augustijns P. Top-down production of drug nanocrystals: Nanosuspension stabilization, miniaturization and transformation into solid products. Int J Pharm. 2008;364:64–75.
Wang H, Xiao Y, Wang H, Sang Z, Han X, Ren S, et al. Development of daidzein nanosuspensions: preparation, characterization, in vitro evaluation, and pharmacokinetic analysis. Int J Pharm [internet]. Elsevier; 2019;566:67–76. Available from. 2019. https://doi.org/10.1016/j.ijpharm.2019.05.051.
Merisko-Liversidge E, Liversidge GG, Cooper ER. Nanosizing: a formulation approach for poorly-water-soluble compounds. Eur J Pharm Sci. 2003;18:113–20.
Dolenc A, Kristl J, Baumgartner S, Planinšek O. Advantages of celecoxib nanosuspension formulation and transformation into tablets. Int J Pharm. 2009;376:204–12.
Gigliobianco MR, Casadidio C, Censi R, Di Martino P. Nanocrystals of poorly soluble drugs: drug bioavailability and physicochemical stability. Pharmaceutics. 2018;10.
Shekhawat P, Pokharkar V. Risk assessment and QbD based optimization of an Eprosartan mesylate nanosuspension: in-vitro characterization, PAMPA and in-vivo assessment. Int J Pharm [internet]. Elsevier; 2019;567:118415. Available from. https://doi.org/10.1016/j.ijpharm.2019.06.006.
Soni S, Patel T, Thakar B, Pandya V, Bharadia P. Nanosuspension: an approach to enhance solubility of drugs. IJPI’s J Pharm Cosmetol. 2012;2:49–63.
Wang C, Cui B, Guo L, Wang A, Zhao X, Wang Y, et al. Fabrication and evaluation of lambda-cyhalothrin nanosuspension by one-step melt emulsification technique. Nanomaterials. 2019;9:1–13.
Elsayed I, Abdelbary AA, Elshafeey AH. Nanosizing of a poorly soluble drug: technique optimization, factorial analysis, and pharmacokinetic study in healthy human volunteers. Int J Nanomedicine. 2014;9:2943–53.
Verma S, Gokhale R, Burgess DJ. A comparative study of top-down and bottom-up approaches for the preparation of micro/nanosuspensions. Int J Pharm. 2009;380:216–22.
Berg JM, Romoser A, Banerjee N, Zebda R, Sayes CM. The relationship between pH and zeta potential of ∼ 30 nm metal oxide nanoparticle suspensions relevant to in vitro toxicological evaluations. Nanotoxicology. 2009;3:276–83.
Ghosh I, Schenck D, Bose S, Liu F, Motto M. Identification of critical process parameters and its interplay with nanosuspension formulation prepared by top down media milling technology – a QbD perspective. Pharm Dev Technol. 2013;18:719–29.
Wang P, Cao X, Chu Y. Ginkgolides-loaded soybean phospholipid-stabilized nanosuspension with improved storage stability and in vivo bioavailability. Colloids Surfaces B Biointerfaces [internet]. Elsevier; 2019;181:910–7. Available from. 2019. https://doi.org/10.1016/j.colsurfb.2019.06.050.
Tashan E, Karakucuk A, Celebi N. Optimization and in vitro evaluation of ziprasidone nanosuspensions produced by a top-down approach. J Drug Deliv Sci Technol [internet]. Elsevier; 2019;52:37–45. Available from. 2019. https://doi.org/10.1016/j.jddst.2019.04.024.
Ain-Ai A, Gupta PK. Effect of arginine hydrochloride and hydroxypropyl cellulose as stabilizers on the physical stability of high drug loading nanosuspensions of a poorly soluble compound. Int J Pharm. 2008;351:282–8.
Niwa T, Miura S, Danjo K. Design of dry nanosuspension with highly spontaneous dispersible characteristics to develop solubilized formulation for poorly water-soluble drugs. Pharm Res. 2011;28:2339–49.
Ghosh I, Bose S, Vippagunta R, Harmon F. Nanosuspension for improving the bioavailability of a poorly soluble drug and screening of stabilizing agents to inhibit crystal growth. Int J Pharm [Internet]. Elsevier B.V.; 2011;409:260–8. Available from: https://doi.org/10.1016/j.ijpharm.2011.02.051
Kobierski S, Ofori-Kwakye K, Müller RH, Keck CM. Resveratrol nanosuspensions for dermal application - production, characterization, and physical stability. Pharmazie. 2009;64:741–7.
Ibrahim AH, Rosqvist E, Smått JH, Ibrahim HM, Ismael HR, Afouna MI, et al. Formulation and optimization of lyophilized nanosuspension tablets to improve the physicochemical properties and provide immediate release of silymarin. Int J Pharm [internet]. Elsevier; 2019;563:217–27. Available from. https://doi.org/10.1016/j.ijpharm.2019.03.064.
Shariare MH, Sharmin S, Jahan I, Reza HM, Mohsin K. The impact of process parameters on carrier free paracetamol nanosuspension prepared using different stabilizers by antisolvent precipitation method. J Drug Deliv Sci Technol [internet]. Elsevier; 2018;43:122–8. Available from. https://doi.org/10.1016/j.jddst.2017.10.001.
Bodratti AM, Sarkar B, Alexandridis P. Adsorption of poly(ethylene oxide)-containing amphiphilic polymers on solid-liquid interfaces: Fundamentals and applications. Adv Colloid Interface Sci [Internet]. Elsevier B.V.; 2017;244:132–63. Available from: https://doi.org/10.1016/j.cis.2016.09.003
Guo L, Kang L, Liu X, Lin X, Di D, Wu Y, et al. A novel nanosuspension of andrographolide: Preparation, characterization and passive liver target evaluation in rats. Eur J Pharm Sci [Internet]. Elsevier B.V.; 2017;104:13–22. Available from: https://doi.org/10.1016/j.ejps.2017.03.017
Ibrahim MA, Shazly GA, Aleanizy FS, Alqahtani FY, Elosaily GM. Formulation and evaluation of docetaxel nanosuspensions: in-vitro evaluation and cytotoxicity. Saudi Pharm J [internet]. King Saud University; 2019;27:49–55. Available from. https://doi.org/10.1016/j.jsps.2018.07.018.
Mishra B, Sahoo J, Dixit PK. Formulation and process optimization of naproxen nanosuspensions stabilized by hydroxy propyl methyl cellulose. Carbohydr Polym [Internet]. Elsevier Ltd.; 2015;127:300–8. Available from: https://doi.org/10.1016/j.carbpol.2015.03.077
Oktay AN, Ilbasmis-tamer S, Karakucuk A, Celebi N. Screening of stabilizing agents to optimize flurbiprofen nanosuspensions using experimental design. J Drug Deliv Sci Technol [Internet]. Elsevier B.V.; 2020; Available from: https://doi.org/10.1016/j.jddst.2020.101690.
Choi J-Y, Yoo J, Kwak H-S, Nam B, Lee J. Role of polymeric stabilizers for drug nanocrystal dispersions. Curr Appl Phys - CURR APPL PHYS. 2005;5:472–4.
George M, Ghosh I. Identifying the correlation between drug/stabilizer properties and critical quality attributes (CQAs) of nanosuspension formulation prepared by wet media milling technology. Eur J Pharm Sci [Internet]. Elsevier B.V.; 2013;48:142–52. Available from: https://doi.org/10.1016/j.ejps.2012.10.004
Merisko-Liversidge E, Liversidge GG. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv Drug Deliv Rev [Internet]. Elsevier B.V.; 2011;63:427–40. Available from: https://doi.org/10.1016/j.addr.2010.12.007
Junyaprasert VB, Morakul B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J Pharm Sci [internet]. Elsevier Ltd. 2015;10:13–23. Available from:. https://doi.org/10.1016/j.ajps.2014.08.005.
Rahimi M, Valeh-e-Sheyda P, Rashidi H. Statistical optimization of curcumin nanosuspension through liquid anti-solvent precipitation (LASP) process in a microfluidic platform: box-Behnken design approach. Korean J Chem Eng. 2017;34:3017–27.
Nagaraj K, Narendar D, Kishan V. Development of olmesartan medoxomil optimized nanosuspension using the box–Behnken design to improve oral bioavailability. Drug Dev Ind Pharm. 2017;43:1186–96.
Singare DS, Marella S, Gowthamrajan K, Kulkarni GT, Vooturi R, Rao PS. Optimization of formulation and process variable of nanosuspension: An industrial perspective. Int J Pharm [Internet]. Elsevier B.V.; 2010;402:213–20. Available from: https://doi.org/10.1016/j.ijpharm.2010.09.041
Hao J, Gao Y, Zhao J, Zhang J, Li Q, Zhao Z, et al. Preparation and optimization of resveratrol Nanosuspensions by Antisolvent precipitation using box-Behnken design. AAPS PharmSciTech. 2014;16:118–28.
Kassem MAA, ElMeshad AN, Fares AR. Enhanced solubility and dissolution rate of Lacidipine Nanosuspension: formulation via Antisolvent Sonoprecipitation technique and optimization using box–Behnken design. AAPS PharmSciTech AAPS PharmSciTech. 2017;18:983–96.
Medarević D, Djuriš J, Ibrić S, Mitrić M, Kachrimanis K. Optimization of formulation and process parameters for the production of carvedilol nanosuspension by wet media milling. Int J Pharm. 2018;540:150–61.
Gadade DD, Pekamwar SS, Lahoti SR, Patni SD, Sarode MC. Etodolak’ın ko-kristalizasyonu: Ko-kristalizasyon tahmini, ko-kristal sentezi, katı faz yapı aydınlatma Çalışmaları ve in vitro İlaç salımı. Marmara Pharm J. 2017;21:78–88.
Gulsun T, Borna SE, Vural I, Sahin S. Preparation and characterization of furosemide nanosuspensions. J Drug Deliv Sci Technol [internet]. Elsevier; 2018;45:93–100. Available from. https://doi.org/10.1016/j.jddst.2018.03.005.
Shariare MH, Altamimi MA, Marzan AL, Tabassum R, Jahan B, Reza HM, et al. In vitro dissolution and bioavailability study of furosemide nanosuspension prepared using design of experiment (DoE). Saudi Pharm J [internet]. King Saud University; 2019;27:96–105. Available from. https://doi.org/10.1016/j.jsps.2018.09.002.
Liu Q, Mai Y, Gu X, Zhao Y, Di X, Ma X, et al. A wet-milling method for the preparation of cilnidipine nanosuspension with enhanced dissolution and oral bioavailability. J Drug Deliv Sci Technol [internet]. Elsevier; 2020;55:101371. Available from. 2020. https://doi.org/10.1016/j.jddst.2019.101371.
Zhang J, Huang Y, Liu D, Gao Y, Qian S. Preparation of apigenin nanocrystals using supercritical antisolvent process for dissolution and bioavailability enhancement. Eur J Pharm Sci [Internet]. 2013;48:740–7. Available from:. https://doi.org/10.1016/j.ejps.2012.12.026.
Azimullah S, Vikrant , Sudhakar C, Kumar P, Patil A, Md. Usman MR, et al. Nanosuspensions as a promising approach to enhance bioavailability of poorly soluble drugs : An update. J Drug Deliv Ther 2019;9:574–582.
Jacob S, Nair AB, Shah J. Emerging role of nanosuspensions in drug delivery systems. Biomater res [internet]. Biomaterials Research. 2020;24:1–16 Available from: http://link.springer.com/article/10.1186/s40824-020-0184-8?utm_source=researcher_app&utm_medium=referral&utm_campaign=RESR_MRKT_Researcher_inbound.
Mosharraf M, Nyström C. The effect of particle size and shape on the surface specific dissolution rate of microsized practically insoluble drugs. Int J Pharm. 1995;122:35–47.
Acknowledgements
This study was supported by a grant from The Scientific and Technological Research Council of Turkey (TUBITAK, 118S718). The correspondence author would like to thank Gazi University Faculty of Pharmacy student Ayca Nur Kondiloglu for studying devotedly as the scholar of the project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 318 kb)
Rights and permissions
About this article
Cite this article
Karakucuk, A., Celebi, N. Investigation of Formulation and Process Parameters of Wet Media Milling to Develop Etodolac Nanosuspensions. Pharm Res 37, 111 (2020). https://doi.org/10.1007/s11095-020-02815-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-020-02815-x