Abstract
Purpose
To prepare an oligo(lactic acid)8-rapamycin prodrug (o(LA)8-RAP)-loaded poly(ethylene glycol)-block-poly(lactic acid) (PEG-b-PLA) micelle for injection and characterize its compatibility and performance versus a RAP-loaded PEG-b-PLA micelle for injection in vitro and in vivo.
Methods
Monodisperse o(LA)8 was coupled on RAP at the C-40 via DCC/DMAP chemistry, and conversion of o(LA)8-RAP prodrug into RAP was characterized in vitro. Physicochemical properties of o(LA)8-RAP- and RAP-loaded PEG-b-PLA micelles and their antitumor efficacies in a syngeneic 4 T1 breast tumor model were compared.
Results
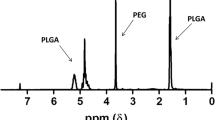
Synthesis of o(LA)8-RAP prodrug was confirmed by 1H NMR and mass spectroscopy. The o(LA)8-RAP prodrug underwent conversion in PBS and rat plasma by backbiting and esterase-mediated cleavage, respectively. O(LA)8-RAP-loaded PEG-b-PLA micelles increased water solubility of RAP equivalent to 3.3 mg/ml with no signs of precipitation. Further, o(LA)8-RAP was released more slowly than RAP from PEG-b-PLA micelles. With added physical stability, o(LA)8-RAP-loaded PEG-b-PLA micelles significantly inhibited tumor growth relative to RAP-loaded PEG-b-PLA micelles in 4 T1 breast tumor-bearing mice without signs of acute toxicity.
Conclusions
An o(LA)8-RAP-loaded PEG-b-PLA micelle for injection is more stable than a RAP-loaded PEG-b-PLA micelle for injection, and o(LA)8-RAP converts into RAP rapidly in rat plasma (t1/2 = 1 h), resulting in antitumor efficacy in a syngeneic 4 T1 breast tumor model.








Similar content being viewed by others
Abbreviations
- ACN:
-
Acetonitrile
- Bn:
-
Benzyl
- CH2Cl2 :
-
Methylene chloride
- DCC:
-
1,3-dicyclohexylcarbodiimide
- DMAP:
-
4-dimethylaminopyridine
- EtOAc:
-
Ethyl acetate
- HF/Pyr:
-
Hydrogen fluoride/pyridine
- mTOR:
-
Mammalian target of rapamycin
- Na2SO4 :
-
Sodium sulphate
- NaHCO3 :
-
Sodium bicarbonate
- O(LA)n :
-
Oligo(lactic acid)n
- O(LA)n-RAP:
-
Oligo(lactic acid)n-rapamycin
- PBS:
-
Phosphate buffered saline
- Pd/C:
-
Palladium on carbon
- PEG-b-PLA:
-
Poly(ethylene glycol)-block-poly(lactic acid)
- RAP:
-
Rapamycin
- Sn(Oct)2 :
-
Tin(II)-ethylhexanoate
- TES:
-
Triethylsilyl ether
- THF:
-
Tetrahydrofuran
- TLC:
-
Thin layer chromatography
References
Zoncu R, Efeyan A, Sabatini DM. MTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12(1):21–35.
Dancey J. mTOR signaling and drug development in cancer. Nat Rev Clin Oncol. 2010;7:209–10.
Zheng Y, Jiang Y. mTOR inhibitors at a glance. Mol Cell Pharmacol. 2015;7(2):15–20.
Lin T, Leung C, Nguyen K, Figlin RA. Mammalian target of rapamycin (mTOR) inhibitors in solid tumours. Cinical Pharm. 2016;8(3):1–23.
Forrest ML, Won CY, Malick AW, Kwon GS. In vitro release of the mTOR inhibitor rapamycin from poly(ethylene glycol)-b-poly(ε-caprolactone) micelles. J Control Release. 2006;110(2):370–7.
Yatscoff RW, Wang P, Chan K, Hicks D, Zimmerman J. Rapamycin: distribution, pharmacokinetics, and therapeutic range investigations. Ther Drug Monit. 1995;17:666–71.
Hartford CM, Ratain MJ. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clin Pharmacol Ther. 2007;82(4):381–8.
Rini BI. Temsirolimus, an inhibitor of mammalian target of rapamycin. Clin Cancer Res. 2008;14(5):1286–90.
Iacovelli R, Santoni M, Verzoni E, Grassi P, Testa I, De Braud F, et al. Everolimus and temsirolimus are not the same second-line in metastatic renal cell carcinoma. A systematic review and meta-analysis of literature data. Clin Genitourin Cancer. 2015;13(2):137–41.
Soefje SA, Karnad A, Brenner AJ. Common toxicities of mammalian target of rapamycin inhibitors. Target Oncol. 2011;6(2):125–9.
Raymond E, Alexandre J, Faivre S, Vera K, Materman E, Boni J, et al. Safety and pharmacokinetics of escalated doses of weekly intravenous infusion of CCI-779, a novel mTOR inhibitor, in patients with cancer. J Clin Oncol. 2004;22(12):2336–47.
Yáñez JA, Forrest ML, Ohgami Y, Kwon GS, Davies NM. Pharmacometrics and delivery of novel nanoformulated PEG-b-poly(ε- caprolactone) micelles of rapamycin. Cancer Chemother Pharmacol. 2008;61(1):133–44.
Xu W, Ling P, Zhang T. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J Drug Deliv. 2013;2013:1–15.
Houdaihed L, Evans JC, Allen C. Overcoming the road blocks: advancement of block copolymer micelles for cancer therapy in the clinic. Mol Pharm. 2017;14(8):2503–17.
Shin DH, Tam YT, Kwon GS. Polymeric micelle nanocarriers in cancer research. Front Chem Sci Eng. 2016;10(3):348–59.
Cho H, Gao J, Kwon GS. PEG-b-PLA micelles and PLGA-b-PEG-b-PLGA sol–gels for drug delivery. J Control Release. 2016;240:191–201.
Chandler D. Interfaces and the driving force of hydrophobic assembly. Nature. 2005;437(7059):640–7.
Riley T, Stolnik S, Heald CR, Xiong CD, Garnett MC, Illum L, et al. Physicochemical evaluation of nanoparticles assembled from poly(lactic acid)-poly(ethylene glycol) (PLA-PEG) block copolymers as drug delivery vehicles. Langmuir. 2001;17(11):3168–74.
Owen SC, Chan DPY, Shoichet MS. Polymeric micelle stability. Nano Today. 2012;7(1):53–65.
Cabral H, Miyata K, Osada K, Kataoka K. Block copolymer micelles in nanomedicine applications. Chem Rev. 2018;118(14):6844–92.
Tam YT, Gao J, Kwon GS. Oligo(lactic acid)n-paclitaxel prodrugs for poly(ethylene glycol)-block-poly(lactic acid) micelles: loading, release, and backbiting conversion for anticancer activity. J Am Chem Soc. 2016;138(28):8674–7.
Tam YT, Huang C, Poellmann M, Kwon GS. Stereocomplex prodrugs of oligo(lactic acid)n-gemcitabine in poly(ethylene glycol)- block-poly(d, l -lactic acid) micelles for improved physical stability and enhanced antitumor efficacy. ACS Nano. 2018;12(7):7406–14.
De Jong SJ, Van Dijk-Wolthuis WNE, Kettenes-Van Den Bosch JJ, PJW S, Hennink WE. Monodisperse enantiomeric lactic acid oligomers: preparation, characterization, and stereocomplex formation. Macromolecules. 1998;31(19):6397–402.
Takizawa K, Nulwala H, Hu J, Yoshinaga K, Hawker CJ. Molecularly defined (L)-lactic acid oligomers and polymers: synthesis and characterization. J Poly Sci. 2008;46:5977–90.
Kaihara S, Matsumura S, Mikos AG, Fisher JP. Synthesis of poly(L-lactide) and polyglycolide by ring-opening polymerization. Nat Protoc. 2007;2(11):2667–71.
Van Nostrum CF, Veldhuis TFJ, Bos GW, Hennink WE. Hydrolytic degradation of oligo(lactic acid): a kinetic and mechanistic study. Polymer. 2004;45(20):6779–87.
Wang H, Zheng X, Cai Z, Yu O, Zheng S, Zhu T. Synthesis and evaluation of an injectable everolimus prodrug. Bioorganic Med Chem Lett. 2017;27(5):1175–8.
Tai W, Chen Z, Barve A, Peng Z, Cheng K. A novel rapamycin-polymer conjugate based on a new poly(ethylene glycol) multiblock copolymer. Pharm Res. 2014;31(3):706–19.
Woo HN, Chung HK, Ju EJ, Jung J, Kang H-W, Lee S-W, et al. Preclinical evaluation of injectable sirolimus formulated with polymeric nanoparticle for cancer therapy. Int J Nanomedicine. 2012;7:2197–208.
Meng LH, Zheng XS. Toward rapamycin analog (rapalog)-based precision cancer therapy. Acta Pharmacol Sin. 2015;36(10):1163–9.
Liederer BM, Borchardt RT. Enzymes involved in the bioconversion of ester-based prodrugs. J Pharm Sci. 2006;95(6):1177–95.
Tam YT, Shin DH, Chen KE, Kwon GS. Poly(ethylene glycol)-block-poly(D,L-lactic acid) micelles containing oligo (lactic acid)8-paclitaxel prodrug: in vivo conversion and antitumor efficacy. J Control Release. 2019;298:186–93.
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editor: Joshua Reineke
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 565 kb)
Rights and permissions
About this article
Cite this article
Tam, Y.T., Repp, L., Ma, ZX. et al. Oligo(Lactic Acid)8-Rapamycin Prodrug-Loaded Poly(Ethylene Glycol)-block-Poly(Lactic Acid) Micelles for Injection. Pharm Res 36, 70 (2019). https://doi.org/10.1007/s11095-019-2600-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-019-2600-0