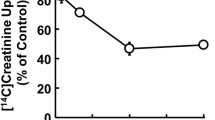
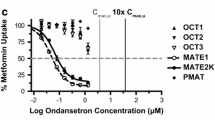
Abstract
Purpose
The organic cation transporters (OCTs) and multidrug and toxin extrusions (MATEs), located in the basolateral and apical membrane of proximal tubular cells respectively, are crucial determinants of renal elimination and/or toxicity of cationic drugs such as cisplatin. The purpose of this study was to discover selective OCT inhibitors over MATEs, and explore their potential to protect against cisplatin-induced nephrotoxicity that is clinically common.
Methods
The inhibition by select compounds on the uptake of the probe substrate metformin was assessed in HEK293 cells overexpressing human OCT2, OCT1, MATE1, MATE2-K, and mouse Oct2, Oct1, and Mate1. Furthermore, the effects of carvedilol on organic cation transporter-mediated cellular and renal accumulation of metformin and cisplatin, and particularly the toxicity associated with cisplatin, were investigated in HEK293 cells and mice.
Results
Five selective OCT inhibitors were identified through the screening of forty-one drugs previously reported as the inhibitors of OCTs and/or MATEs. Among them, carvedilol showed the most selectivity on OCTs over MATEs (IC50: 3.6 μM for human OCT2, 103 μM for human MATE1 and 202 μM for human MATE2-K) in the cellular assays in vitro, with the selectivity in mice as well. Moreover, carvedilol treatment could significantly decrease cisplatin accumulation and ameliorate its toxicity both in vitro in cells and in vivo in mouse kidney.
Conclusions
Our data indicate that selective inhibition of OCTs by carvedilol may protect from cisplatin-induced nephrotoxicity by restraining the cellular entry of cisplatin via OCTs, while having no impact on its elimination through MATEs.






Similar content being viewed by others
Abbreviations
- AUC:
-
Area under curve
- Cmax :
-
The maximum concentration
- DDI:
-
Drug-drug interaction
- H&E staining:
-
Haematoxylin and Eosin staining
- IC50 :
-
The half maximal inhibitory concentration
- MATE:
-
Multidrug and toxin extrusions
- MPP+ :
-
1-methyl-4-phenylpyridinium
- OCT:
-
Organic cation transporters
- T1/2 :
-
Half life
- TEA:
-
Tetraethyl ammonium
- Tmax :
-
Time of the maxium concentration
References
Gorboulev V, et al. Cloning and characterization of two human polyspecific organic cation transporters. DNA Cell Biol. 1997;16(7):871–81.
Grundemann D, et al. Selective substrates for non-neuronal monoamine transporters. Mol Pharmacol. 1999;56(1):1–10.
Kimura N, et al. Metformin is a superior substrate for renal organic cation transporter OCT2 rather than hepatic OCT1. Drug Metab Pharmacokinet. 2005;20(5):379–86.
Muller F, et al. Role of organic cation transporter OCT2 and multidrug and toxin extrusion proteins MATE1 and MATE2-K for transport and drug interactions of the antiviral lamivudine. Biochem Pharmacol. 2013;86(6):808–15.
Jung N, et al. Organic cation transporters OCT1 and OCT2 determine the accumulation of lamivudine in CD4 cells of HIV-infected patients. Infection. 2013;41(2):379–85.
Burger H, et al. Differential transport of platinum compounds by the human organic cation transporter hOCT2 (hSLC22A2). Br J Pharmacol. 2010;159(4):898–908.
Urakami Y, et al. Creatinine transport by basolateral organic cation transporter hOCT2 in the human kidney. Pharm Res. 2004;21(6):976–81.
Zhang Y, et al. Impact on creatinine renal clearance by the interplay of multiple renal transporters: a case study with INCB039110. Drug Metab Dispos. 2015;43(4):485–9.
Motohashi H, et al. Gene expression levels and immunolocalization of organic ion transporters in the human kidney. J Am Soc Nephrol. 2002;13(4):866–74.
Jonker JW, et al. Deficiency in the organic cation transporters 1 and 2 (Oct1/Oct2 [Slc22a1/Slc22a2]) in mice abolishes renal secretion of organic cations. Mol Cell Biol. 2003;23(21):7902–8.
Filipski KK, et al. Contribution of organic cation transporter 2 (OCT2) to cisplatin-induced nephrotoxicity. Clin Pharmacol Ther. 2009;86(4):396–402.
Iwata K, et al. Effects of genetic variants in SLC22A2 organic cation transporter 2 and SLC47A1 multidrug and toxin extrusion 1 transporter on cisplatin-induced adverse events. Clin Exp Nephrol. 2012;16(6):843–51.
Nakamura T, et al. Disruption of multidrug and toxin extrusion MATE1 potentiates cisplatin-induced nephrotoxicity. Biochem Pharmacol. 2010;80(11):1762–7.
Li Q, et al. Ondansetron can enhance cisplatin-induced nephrotoxicity via inhibition of multiple toxin and extrusion proteins (MATEs). Toxicol Appl Pharmacol. 2013;273(1):100–9.
Yonezawa A, et al. Cisplatin and oxaliplatin, but not carboplatin and nedaplatin, are substrates for human organic cation transporters (SLC22A1-3 and multidrug and toxin extrusion family). J Pharmacol Exp Ther. 2006;319(2):879–86.
Yokoo S, et al. Differential contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem Pharmacol. 2007;74(3):477–87.
Minami T, et al. Accumulation of platinum in the intervertebral discs and vertebrae of ovarian tumor-bearing patients treated with cisplatin. Biol Trace Elem Res. 1994;42(3):253–7.
Wang EJ, et al. Validation of putative genomic biomarkers of nephrotoxicity in rats. Toxicology. 2008;246(2–3):91–100.
Minematsu T, Giacomini KM. Interactions of tyrosine kinase inhibitors with organic cation transporters and multidrug and toxic compound extrusion proteins. Mol Cancer Ther. 2011;10(3):531–9.
Vaidya VS, et al. Kidney injury molecule-1 outperforms traditional biomarkers of kidney injury in preclinical biomarker qualification studies. Nat Biotechnol. 2010;28(5):478–85.
Madias NE, Harrington JT. Platinum nephrotoxicity. Am J Med. 1978;65(2):307–14.
Yonezawa A, et al. Association between tubular toxicity of cisplatin and expression of organic cation transporter rOCT2 (Slc22a2) in the rat. Biochem Pharmacol. 2005;70(12):1823–31.
Sleijfer DT, et al. The protective potential of the combination of verapamil and cimetidine on cisplatin-induced nephrotoxicity in man. Cancer. 1987;60(11):2823–8.
Katsuda H, et al. Protecting cisplatin-induced nephrotoxicity with cimetidine does not affect antitumor activity. Biol Pharm Bull. 2010;33(11):1867–71.
Zhang J, Zhou W. Ameliorative effects of SLC22A2 gene polymorphism 808 G/T and cimetidine on cisplatin-induced nephrotoxicity in Chinese cancer patients. Food Chem Toxicol. 2012;50(7):2289–93.
Sprowl JA, et al. Conjunctive therapy of cisplatin with the OCT2 inhibitor cimetidine: influence on antitumor efficacy and systemic clearance. Clin Pharmacol Ther. 2013;94(5):585–92.
Dorr RT, Soble MJ. Cimetidine enhances cisplatin toxicity in mice. J Cancer Res Clin Oncol. 1988;114(1):1–2.
Ito S, et al. Competitive inhibition of the luminal efflux by multidrug and toxin extrusions, but not basolateral uptake by organic cation transporter 2, is the likely mechanism underlying the pharmacokinetic drug-drug interactions caused by cimetidine in the kidney. J Pharmacol Exp Ther. 2012;340(2):393–403.
Wittwer MB, et al. Discovery of potent, selective multidrug and toxin extrusion transporter 1 (MATE1, SLC47A1) inhibitors through prescription drug profiling and computational modeling. J Med Chem. 2013;56(3):781–95.
Masuda S, et al. Identification and functional characterization of a new human kidney-specific H+/organic cation antiporter, kidney-specific multidrug and toxin extrusion 2. J Am Soc Nephrol. 2006;17(8):2127–35.
Ruffolo RR, Feuerstein GZ. Carvedilol case history: the discovery and development of the first beta-blocker for the treatment of congestive heart failure. Expert Opin Drug Discovery. 2006;1(1):85–9.
Morgan T. Clinical pharmacokinetics and pharmacodynamics of carvedilol. Clin Pharmacokinet. 1994;26(5):335–46.
Stahl E, et al. Carvedilol stereopharmacokinetics in rats: affinities to blood constituents and tissues. Arch Pharm (Weinheim). 1993;326(9):529–33.
Nakamichi N, et al. Involvement of carnitine/organic cation transporter OCTN1/SLC22A4 in gastrointestinal absorption of metformin. J Pharm Sci. 2013;102(9):3407–17.
Wu X, et al. Structural and functional characteristics and tissue distribution pattern of rat OCTN1, an organic cation transporter, cloned from placenta. Biochim Biophys Acta. 2000;1466(1–2):315–27.
Higgins JW, Bedwell DW, Zamek-Gliszczynski MJ. Ablation of both organic cation transporter (OCT)1 and OCT2 alters metformin pharmacokinetics but has no effect on tissue drug exposure and pharmacodynamics. Drug Metab Dispos. 2012;40(6):1170–7.
Li Q, et al. Deficiency of multidrug and toxin extrusion 1 enhances renal accumulation of paraquat and deteriorates kidney injury in mice. Mol Pharm. 2011;8(6):2476–83.
Carvalho Rodrigues MA, et al. Carvedilol efficiently protects kidneys without affecting the antitumor efficacy of cisplatin in mice. Chem Biol Interact. 2013;206(1):90–9.
Carvalho Rodrigues MA, et al. Carvedilol protects against apoptotic cell death induced by cisplatin in renal tubular epithelial cells. J Toxic Environ Health A. 2012;75(16–17):981–90.
Rodrigues MA, et al. Carvedilol protects against cisplatin-induced oxidative stress, redox state unbalance and apoptosis in rat kidney mitochondria. Chem Biol Interact. 2011;189(1–2):45–51.
Ruffolo RR Jr, Feuerstein GZ. Pharmacology of carvedilol: rationale for use in hypertension, coronary artery disease, and congestive heart failure. Cardiovasc Drugs Ther. 1997;11(Suppl 1):247–56.
Bachmakov I, et al. Interaction of beta-blockers with the renal uptake transporter OCT2. Diabetes Obes Metab. 2009;11(11):1080–3.
Acknowledgments and Disclosures
The present study was supported by the National Institute of General Medical Sciences of the US National Institutes of Health (NIH) [R01GM099742] and by the US Food and Drug Administration (FDA) [U01FD004320]. Dr. Dong Guo is an M-CERSI Scholar (FDA 1U01FD005946). Dr. Yan Shu is a co-founder for and owns equity in Optivia Biotechnology.
Author information
Authors and Affiliations
Contributions
Designed Research: Yan Shu, James E. polli, Dong Guo.
Performed Research: Dong Guo, Hong Yang, Qing Li, Hyo Jung Bae, Sujuan Zeng, Tong Su.
Analyzed Data: Dong Guo, Hong Yang, Qing Li, Hyo Jung Bae, Yan Shu.
Wrote Manuscript: Dong Guo, Yan Shu.
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, D., Yang, H., Li, Q. et al. Selective Inhibition on Organic Cation Transporters by Carvedilol Protects Mice from Cisplatin-Induced Nephrotoxicity. Pharm Res 35, 204 (2018). https://doi.org/10.1007/s11095-018-2486-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-018-2486-2