Abstract
Purpose
In view of pediatric drug development, juvenile animal studies are gaining importance. However, data on drug metabolizing capacities of juvenile animals are scarce, especially in non-rodent species. Therefore, we aimed to characterize the in vitro biotransformation of four human CYP450 substrates and one UGT substrate in the livers of developing Göttingen minipigs.
Methods
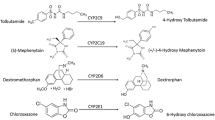
Liver microsomes from late fetal, Day 1, Day 3, Day 7, Day 28, and adult male and female Göttingen minipigs were incubated with a cocktail of CYP450 substrates, including phenacetin, tolbutamide, dextromethorphan, and midazolam. The latter are probe substrates for human CYP1A2, CYP2C9, CYP2D6, and CYP3A4, respectively. In addition, the UGT multienzyme substrate (from the UGT-GloTM assay), which is glucuronidated by several human UGT1A and UGT2B enzymes, was also incubated with the porcine liver microsomes.
Results
For all tested substrates, drug metabolism significantly rose postnatally. At one month of age, 60.5 and 75.4% of adult activities were observed for acetaminophen and dextrorphan formations, respectively, while 35.4 and 43.2% of adult activities were present for 4-OH-tolbutamide and 1’-OH-midazolam formations. Biotransformation of phenacetin was significantly higher in 28-day-old and adult females compared with males.
Conclusions
Maturation of metabolizing capacities occurred postnatally, as described in man.






Similar content being viewed by others
Abbreviations
- CYP450:
-
Cytochrome P450 enzymes
- DGA:
-
Days of gestational age
- HLM:
-
Human liver microsomes
- KPO4 :
-
Potassium phosphate
- LLOQ:
-
Lower limit of quantification
- MLM:
-
Minipig liver microsomes
- mol/min/mg MP:
-
Moles of metabolite formed per minute per milligram of microsomal protein
- MP:
-
Microsomal protein
- PBS:
-
Phosphate buffered saline
- PND:
-
Postnatal day
- RLU:
-
Relative light unit
- RT:
-
Room temperature
- TBS:
-
Tris buffered saline
- UDPGA:
-
Uridine-5’-diphospho-glucuronic acid
- UGT:
-
Uridine diphosphate glucuronosyltransferase
- Vmax :
-
Maximal velocity
References
Carleer J, Karres J. Juvenile animal studies and pediatric drug development: a European regulatory perspective. Birth Defects Res Part B, Dev Reproduct Toxicol. 2011;92(4):254–60.
Baldrick P. Juvenile animal testing in drug development--is it useful? Regul Toxicol Pharmacol. 2010;57(2-3):291–9.
Bode G, Clausing P, Gervais F, Loegsted J, Luft J, Nogues V, et al. The utility of the minipig as an animal model in regulatory toxicology. J Pharmacol Toxicol Methods. 2010;62(3):196–220.
Hayes AW. Principles and methods of toxicology. 4th ed. London: Taylor & Francis; 2001.
Puccinelli E, Gervasi PG, Longo V. Xenobiotic metabolizing cytochrome P450 in pig, a promising animal model. Curr Drug Metab. 2011;12(6):507–25.
Li J, Liu Y, Zhang JW, Wei H, Yang L. Characterization of hepatic drug-metabolizing activities of Bama miniature pigs (Sus scrofa domestica): comparison with human enzyme analogs. Comp Med. 2006;56(4):286–90.
Hu SX. Impact of age on hepatic cytochrome P450 of domestic male Camborough-29 pigs. J Vet Pharmacol Ther. 2014.
Achour B, Barber J, Rostami-Hodjegan A. Cytochrome p450 pig liver pie: determination of individual cytochrome p450 isoform contents in microsomes from two pig livers using liquid chromatography in conjunction with mass spectroscopy. Drug Metab Dispos. 2011;39(11):2130–4.
Messina A, Chirulli V, Gervasi PG, Longo V. Purification, molecular cloning, heterologous expression and characterization of pig CYP1A2. Xenobiotica. 2008;38(12):1453–70.
Skaanild MT, Friis C. Analyses of CYP2C in porcine microsomes. Basic Clin Pharmacol Toxicol. 2008;103(5):487–92.
Anzenbacher P, Soucek P, Anzenbacherova E, Gut I, Hruby K, Svoboda Z, et al. Presence and activity of cytochrome P450 isoforms in minipig liver microsomes. comparison with human liver samples. Drug Metab Dispos. 1998;26(1):56–9.
Soucek P, Zuber R, Anzenbacherova E, Anzenbacher P, Guengerich FP. Minipig cytochrome P450 3A, 2A and 2C enzymes have similar properties to human analogs. BMC Pharmacol. 2001;1:11.
Wester MR, Lasker JM, Johnson EF, Raucy JL. CYP2C19 participates in tolbutamide hydroxylation by human liver microsomes. Drug Metab Dispos. 2000;28(3):354–9.
Skaanild MT, Friis C. Cytochrome P450 sex differences in minipigs and conventional pigs. Pharmacol Toxicol. 1999;85(4):174–80.
Skaanild MT, Friis C. Is cytochrome P450 CYP2D activity present in pig liver? Pharmacol Toxicol. 2002;91(4):198–203.
Jurima-Romet M, Casley WL, Leblanc CA, Nowakowska M. Evidence for the catalysis of dextromethorphan O-demethylation by a CYP2D6-like enzyme in pig liver. Toxicol In Vitro : Int J Publ Assoc BIBRA. 2000;14(3):253–63.
Sakuma T, Shimojima T, Miwa K, Kamataki T. Cloning CYP2D21 and CYP3A22 cDNAs from liver of miniature pigs. Drug Metab Dispos. 2004;32(4):376–8.
Murayama N, Kaneko N, Horiuchi K, Ohyama K, Shimizu M, Ito K, et al. Cytochrome P450-dependent drug oxidation activity of liver microsomes from Microminipigs, a possible new animal model for humans in non-clinical studies. Drug Metab Pharmacokinetics. 2009;24(4):404–8.
Miyake Y, Mayumi K, Jinno H, Tanaka-Kagawa T, Narimatsu S, Hanioka N. cDNA cloning and functional analysis of minipig uridine diphosphate-glucuronosyltransferase 1A1. Biol Pharm Bull. 2013;36(3):452–61.
Higashi E, Ando A, Iwano S, Murayama N, Yamazaki H, Miyamoto Y. Hepatic microsomal UDP-glucuronosyltransferase (UGT) activities in the microminipig. Biopharm Drug Dispos. 2014;35(6):313–20.
Heckel T, Schmucki R, Berrera M, Ringshandl S, Badi L, Steiner G, et al. Functional analysis and transcriptional output of the Gottingen minipig genome. BMC Genomics. 2015;16(1):932.
Van Peer E, De Bock L, Boussery K, Van Bocxlaer J, Casteleyn C, Van Ginneken C, et al. Age-related differences in CYP3A abundance and activity in the liver of the Gottingen Minipig. Basic Clin Pharmacol Toxicol. 2015;117(5):350–7.
Fisher MB, Campanale K, Ackermann BL, VandenBranden M, Wrighton SA. In vitro glucuronidation using human liver microsomes and the pore-forming peptide alamethicin. Drug Metab Dispos. 2000;28(5):560–6.
Cazeneuve C, Pons G, Rey E, Treluyer JM, Cresteil T, Thiroux G, et al. Biotransformation of caffeine in human liver microsomes from foetuses, neonates, infants and adults. Br J Clin Pharmacol. 1994;37(5):405–12.
Sonnier M, Cresteil T. Delayed ontogenesis of CYP1A2 in the human liver. Eur J Biochem. 1998;251(3):893–8.
Skaanild MT, Friis C. Characterization of the P450 system in Gottingen minipigs. Pharmacol Toxicol. 1997;80 Suppl 2:28–33.
Kojima M, Sekimoto M, Degawa M. A novel gender-related difference in the constitutive expression of hepatic cytochrome P4501A subfamily enzymes in Meishan pigs. Biochem Pharmacol. 2008;75(5):1076–82.
Rasmussen MK, Zamaratskaia G, Ekstrand B. Gender-related differences in cytochrome P450 in porcine liver--implication for activity, expression and inhibition by testicular steroids. Reprod Domestic Anim = Zuchthygiene. 2011;46(4):616–23.
Ou-Yang DS, Huang SL, Wang W, Xie HG, Xu ZH, Shu Y, et al. Phenotypic polymorphism and gender-related differences of CYP1A2 activity in a Chinese population. Br J Clin Pharmacol. 2000;49(2):145–51.
Parkinson A, Mudra DR, Johnson C, Dwyer A, Carroll KM. The effects of gender, age, ethnicity, and liver cirrhosis on cytochrome P450 enzyme activity in human liver microsomes and inducibility in cultured human hepatocytes. Toxicol Appl Pharmacol. 2004;199(3):193–209.
Treluyer JM, Gueret G, Cheron G, Sonnier M, Cresteil T. Developmental expression of CYP2C and CYP2C-dependent activities in the human liver: in-vivo/in-vitro correlation and inducibility. Pharmacogenetics. 1997;7(6):441–52.
Koukouritaki SB, Manro JR, Marsh SA, Stevens JC, Rettie AE, McCarver DG, et al. Developmental expression of human hepatic CYP2C9 and CYP2C19. J Pharmacol Exp Ther. 2004;308(3):965–74.
Kojima M, Degawa M. Sex differences in constitutive mRNA levels of CYP2B22, CYP2C33, CYP2C49, CYP3A22, CYP3A29 and CYP3A46 in the pig liver: comparison between Meishan and Landrace pigs. Drug Metab Pharmacokinetics. 2016;31(3):185–92.
Treluyer JM, Jacqz-Aigrain E, Alvarez F, Cresteil T. Expression of CYP2D6 in developing human liver. Eur J Biochem. 1991;202(2):583–8.
Stevens JC, Marsh SA, Zaya MJ, Regina KJ, Divakaran K, Le M, et al. Developmental changes in human liver CYP2D6 expression. Drug Metab Dispos. 2008;36(8):1587–93.
Blake MJ, Gaedigk A, Pearce RE, Bomgaars LR, Christensen ML, Stowe C, et al. Ontogeny of dextromethorphan O- and N-demethylation in the first year of life. Clin Pharmacol Ther. 2007;81(4):510–6.
Johnson TN, Thomson M. Intestinal metabolism and transport of drugs in children: the effects of age and disease. J Pediatr Gastroenterol Nutr. 2008;47(1):3–10.
Wang H, Tompkins LM. CYP2B6: new insights into a historically overlooked cytochrome P450 isozyme. Curr Drug Metab. 2008;9(7):598–610.
Croom EL, Stevens JC, Hines RN, Wallace AD, Hodgson E. Human hepatic CYP2B6 developmental expression: the impact of age and genotype. Biochem Pharmacol. 2009;78(2):184–90.
Lacroix D, Sonnier M, Moncion A, Cheron G, Cresteil T. Expression of CYP3A in the human liver--evidence that the shift between CYP3A7 and CYP3A4 occurs immediately after birth. Eur J Biochem. 1997;247(2):625–34.
Blanco JG, Harrison PL, Evans WE, Relling MV. Human cytochrome P450 maximal activities in pediatric versus adult liver. Drug Metab Dispos. 2000;28(4):379–82.
Stevens JC, Hines RN, Gu C, Koukouritaki SB, Manro JR, Tandler PJ, et al. Developmental expression of the major human hepatic CYP3A enzymes. J Pharmacol Exp Ther. 2003;307(2):573–82.
Snawder JE, Lipscomb JC. Interindividual variance of cytochrome P450 forms in human hepatic microsomes: correlation of individual forms with xenobiotic metabolism and implications in risk assessment. Regul Toxicol Pharmacol. 2000;32(2):200–9.
Debinski HS, Lee CS, Danks JA, Mackenzie PI, Desmond PV. Localization of uridine 5'-diphosphate-glucuronosyltransferase in human liver injury. Gastroenterology. 1995;108(5):1464–9.
Strassburg CP, Strassburg A, Kneip S, Barut A, Tukey RH, Rodeck B, et al. Developmental aspects of human hepatic drug glucuronidation in young children and adults. Gut. 2002;50(2):259–65.
Alcorn J, McNamara PJ. Pharmacokinetics in the newborn. Adv Drug Deliv Rev. 2003;55(5):667–86.
Isoherranen N, Thummel KE. Drug metabolism and transport during pregnancy: how does drug disposition change during pregnancy and what are the mechanisms that cause such changes? Drug Metab Dispos. 2013;41(2):256–62.
Skaanild MT. Porcine cytochrome P450 and metabolism. Curr Pharm Des. 2006;12(11):1421–7.
ACKNOWLEDGMENTS AND DISCLOSURES
The Applied Veterinary Morphology research group would like to thank Ellegaard Göttingen Minipig A/S for the kind donation of animals. The authors from the University of Antwerp are members of COST Action BM1308 ‘Sharing Advances on Large Animal Models (SALAAM)’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no conflict of interest.
Ethical Approval
The Ethical Committee of Animal Experimentation from the University of Antwerp (Belgium) approved the protocol and use of the animals and research adhered to the ‘Principles of Laboratory Animal Care’ (NIH publication Nr 85-23, revised in 1985).
Rights and permissions
About this article
Cite this article
Van Peer, E., Jacobs, F., Snoeys, J. et al. In vitro Phase I- and Phase II-Drug Metabolism in The Liver of Juvenile and Adult Göttingen Minipigs. Pharm Res 34, 750–764 (2017). https://doi.org/10.1007/s11095-017-2101-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-017-2101-y