Abstract
Purpose
To develop polymeric films containing dual combinations of anti-HIV drug candidate tenofovir, maraviroc and dapivirine for vaginal application as topical microbicides.
Methods
A solvent casting method was used to manufacture the films. Solid phase solubility was used to identify potential polymers for use in the film formulation. Physical and chemical properties (such as water content, puncture strength and in vitro release) and product stability were determined. The bioactivity of the film products against HIV was assessed using the TZM-bl assay and a cervical explant model.
Results
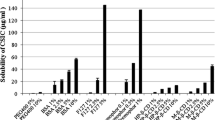
Polymers identified from the solid phase solubility study maintained tenofovir and maraviroc in an amorphous state and prevented drug crystallization. Three combination film products were developed using cellulose polymers and polyvinyl alcohol. The residual water content in all films was <10% (w/w). All films delivered the active agents with release of >50% of film drug content within 30 min. Stability testing confirmed that the combination film products were stable for 12 months at ambient temperature and 6 months under stressed conditions. Antiviral activity was confirmed in TZM-bl and cervical explant models.
Conclusions
Polymeric films can be used as a stable dosage form for the delivery of antiretroviral combinations as microbicides.







Similar content being viewed by others
Abbreviations
- ARVs:
-
Antiretrovirals
- AZT:
-
Zidovudine
- DPV:
-
Dapivirine
- HAART:
-
Highly antiretroviral active therapy
- HEC:
-
Hydroxy ethyl cellulose
- HPMC:
-
Hydroxy propyl methyl cellulose
- IHC:
-
Immunohistochemical
- MVC:
-
Maraviroc
- Na CMC:
-
Carboxy methyl cellulose sodium
- PEG:
-
Poly ethylene glycol
- PVA:
-
Poly vinyl alcohol
- PVP:
-
Poly vinyl pyrrolidone
- SMST:
-
Standard microbicide safety test
- SPE:
-
Solid phase extraction
- STIs:
-
Sexual transmitted infections
- tBAHS:
-
t-Butylammonium bisulfate
- TFA:
-
Triflouro acetic acid
- TFV:
-
Tenofovir
- UHPLC:
-
Ultra high pressure liquid chromatography
References
Shattock RJ, Rosenberg Z. Microbicides: topical prevention against HIV. Cold Spring Harb Perspect Med. 2012;2(2):a007385.
Abdool Karim SS, Baxter C. Overview of microbicides for the prevention of human immunodeficiency virus. Best Pract Res Clin Obstet Gynaecol. 2012;26(4):427–39.
Brechtl JR, Breitbart W, Galietta M, Krivo S, Rosenfeld B. The use of highly active antiretroviral therapy (HAART) in patients with advanced HIV infection: impact on medical, palliative care, and quality of life outcomes. J Pain Symptom Manag. 2001;21(1):41–51.
Rosenberg ZF, Devlin B. Future strategies in microbicide development. Best Pract Res Clin Obstet Gynaecol. 2012;26(4):503–13.
Verma NA, Lee AC, Herold BC, Keller MJ. Topical prophylaxis for HIV prevention in women: becoming a reality. Curr HIV/AIDS Rep. 2011;8(2):104–13.
Lewi P, Heeres J, Arien K, Venkatraj M, Joossens J, Van der Veken P, et al. Reverse transcriptase inhibitors as microbicides. Curr HIV Res. 2012;10(1):27–35.
Dorr P, Westby M, Dobbs S, Griffin P, Irvine B, Macartney M, et al. Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity. Antimicrob Agents Chemother. 2005;49(11):4721–32.
Schader SM, Colby-Germinario SP, Schachter JR, Xu H, Wainberg MA. Synergy against drug-resistant HIV-1 with the microbicide antiretrovirals, dapivirine and tenofovir, in combination. AIDS. 2011;25(13):1585–94.
Schader SM, Oliveira M, Ibanescu RI, Moisi D, Colby-Germinario SP, Wainberg MA. In vitro resistance profile of the candidate HIV-1 microbicide drug dapivirine. Antimicrob Agents Chemother. 2012;56(2):751–6.
Adams JL, Kashuba AD. Formulation, pharmacokinetics and pharmacodynamics of topical microbicides. Best Pract Res Clin Obstet Gynaecol. 2012;26(4):451–62.
Romano J, Malcolm RK, Garg S, Rohan LC, Kaptur PE. Microbicide delivery: formulation technologies and strategies. Curr Opin HIV AIDS. 2008;3(5):558–66.
Nel AM, Mitchnick LB, Risha P, Muungo LT, Norick PM. Acceptability of vaginal film, soft-gel capsule, and tablet as potential microbicide delivery methods among African women. J Womens Health (Larchmt). 2011;20(8):1207–14.
Yoo JW, Acharya G, Lee CH. In vivo evaluation of vaginal films for mucosal delivery of nitric oxide. Biomaterials. 2009;30(23–24):3978–85.
Dobaria NB, Badhan AC, Mashru RC. A novel itraconazole bioadhesive film for vaginal delivery: design, optimization, and physicodynamic characterization. AAPS PharmSciTech. 2009;10(3):951–9.
Dobaria N, Mashru R. Design and in vitro evaluation of a novel bioadhesive vaginal drug delivery system for clindamycin phosphate. Pharm Dev Technol. 2009;15(4):405–14.
Sudeendra BR, Umme H, Gupta RK, Shivakumar HG. Development and characterization of bioadhesive vaginal films of clotrimazole for vaginal candidiasis. Acta Pharm Sci. 2010;52:417–26.
Neurath AR, Strick N, Li YY. Water dispersible microbicidal cellulose acetate phthalate film. BMC Infect Dis. 2003;3:27.
Garg S, Vermani K, Garg A, Anderson RA, Rencher WB, Zaneveld LJ. Development and characterization of bioadhesive vaginal films of sodium polystyrene sulfonate (PSS), a novel contraceptive antimicrobial agent. Pharm Res. 2005;22(4):584–95.
Akil A, Parniak M, Dezzutti C, Moncla B, Cost M, Li M, et al. Development and characterization of a vaginal film containing dapivirine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Deliv Transl Res. 2011;1(3):209–22.
Ham AS, Rohan LC, Boczar A, Yang L, WB K, Buckheit Jr RW. Vaginal film drug delivery of the pyrimidinedione IQP-0528 for the prevention of HIV infection. Pharm Res. 2012;29(7):1897–907.
Chatterjee A, Bhowmik BB, Awasthi D. Prolong release bioadhesive vaginal film of anti-hiv drug (zidovudine): formulation and in vitro evaluation. Int J Pharm Sci Res. 2010;1(3):28–37.
Sassi AB, Cost MR, Cole AL, Cole AM, Patton DL, Gupta P, et al. Formulation development of retrocyclin 1 analog RC-101 as an anti-HIV vaginal microbicide product. Antimicrob Agents Chemother. 2011;55(5):2282–9.
Cole AM, Patton DL, Rohan LC, Cole AL, Cosgrove-Sweeney Y, Rogers NA, et al. The formulated microbicide RC-101 was safe and antivirally active following intravaginal application in pigtailed macaques. PLoS One. 2010;5(11):e15111.
Wei X, Decker JM, Liu H, Zhang Z, Arani RB, Kilby JM, et al. Emergence of resistant human immunodeficiency virus type 1 in patients receiving fusion inhibitor (T-20) monotherapy. Antimicrob Agents Chemother. 2002;46(6):1896–905.
Moncla BJ, Hillier SL. Why nonoxynol-9 may have failed to prevent acquisition of Neisseria gonorrhoeae in clinical trials. Sex Transm Dis. 2005;32(8):491–4.
Moncla BJ, Pryke K, Isaacs CE. Killing of Neisseria gonorrhoeae, Streptococcus agalactiae (group B streptococcus), Haemophilus ducreyi, and vaginal Lactobacillus by 3-O-octyl-sn-glycerol. Antimicrob Agents Chemother. 2008;52(4):1577–9.
Cummins Jr JE, Guarner J, Flowers L, Guenthner PC, Bartlett J, Morken T, et al. Preclinical testing of candidate topical microbicides for anti-human immunodeficiency virus type 1 activity and tissue toxicity in a human cervical explant culture. Antimicrob Agents Chemother. 2007;51(5):1770–9.
Rohan LC, Moncla BJ, Kunjara Na Ayudhya RP, Cost M, Huang Y, Gai F, et al. In vitro and ex vivo testing of tenofovir shows it is effective as an HIV-1 microbicide. PLoS ONE. 2010;5(2):e9310.
Mayer KH, Maslankowski LA, Gai F, El-Sadr WM, Justman J, Kwiecien A, et al. Safety and tolerability of tenofovir vaginal gel in abstinent and sexually active HIV-infected and uninfected women. AIDS. 2006;20(4):543–51.
Nel AM, Smythe SC, Habibi S, Kaptur PE, Romano JW. Pharmacokinetics of 2 dapivirine vaginal microbicide gels and their safety vs. Hydroxyethyl cellulose-based universal placebo gel. J Acquir Immune Defic Syndr. 2010;55(2):161–9.
Neff CP, Kurisu T, Ndolo T, Fox K, Akkina R. A topical microbicide gel formulation of CCR5 antagonist maraviroc prevents HIV-1 vaginal transmission in humanized RAG-hu mice. PLoS ONE. 2011;6(6):e20209.
Veazey RS, Ketas TJ, Dufour J, Moroney-Rasmussen T, Green LC, Klasse PJ, et al. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J Infect Dis. 2010;202(5):739–44.
Hariharan M, Bogue BA. Orally dissolving film strips (ODFS): the final evolution of orally dissolving dosage forms. Drug Deliv Technol. 2009;9(2):24–9.
Raghavan SL, Trividic A, Davis AF, Hadgraft J. Crystallization of hydrocortisone acetate: influence of polymers. Int J Pharm. 2001;212(2):213–21.
Kestur US, Taylor LS. Role of polymer chemistry in influencing crystal growth rates from amorphous felodipine. Crystengcomm. 2010;12(8):2390–7.
ACKNOWLEDGMENTS AND DISCLOSURES
The work presented was supported through a grant from the National Institute of Allergy and Infectious Diseases (NIAID) at the National Institute of Health (AI082639) and the International Partnership for Microbicides (IPM). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Akil, A., Agashe, H., Dezzutti, C.S. et al. Formulation and Characterization of Polymeric Films Containing Combinations of Antiretrovirals (ARVs) for HIV Prevention. Pharm Res 32, 458–468 (2015). https://doi.org/10.1007/s11095-014-1474-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-014-1474-4