ABSTRACT
Purpose
Temozolomide dry powder formulations for inhalation, performed with no excipient or with a lipid or lactose coating, have been evaluated.
Methods
The particle size of raw temozolomide in suspension was reduced by a high-pressure homogenizing technique, and the solvent was evaporated by spray-drying to obtain a dry powder. The physicochemical properties of this powder were evaluated and included its crystalline state, thermal properties, morphology, particle size and moisture and drug content, and these properties were determined by X-ray powder diffraction, differential scanning calorimetry, scanning electron microscopy, laser light scattering, thermogravimetric analysis and high-performance liquid chromatography, respectively. The aerodynamic properties and release profiles were also evaluated using a multistage liquid impinger and a modified USP type 2 dissolution apparatus adapted for inhaler products, respectively.
Results
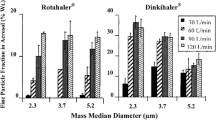
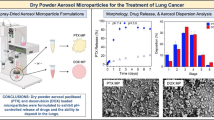
The dry powder inhalation formulations had a high temozolomide content that ranged from 70% to 100% in the crystalline state and low moisture content. Aerodynamic evaluations showed high fine-particle fractions of up to 51% related to the metered dose. The dissolution profile revealed a similarly fast temozolomide release from the formulations.
Conclusions
Dry temozolomide powder formulations, based on the use of acceptable excipients for inhalation and showing good dispersion properties, represent an attractive alternative for use in local lung cancer therapy.






Similar content being viewed by others
Abbreviations
- DLPC:
-
1,2-dilauroyl-sn-glycero-3-phosphocholine
- DMPC:
-
1,2-dimyristoyl-sn-glycero-3-phosphocholine
- DPI:
-
dry powder inhaler
- DPPC:
-
dipalmitoyl phosphatidylcholine
- DSC:
-
differential scanning calorimetry
- FPD:
-
fine particle dose
- FPF:
-
fine particle fraction
- HPH:
-
high-pressure homogenizing
- HPLC:
-
high-performance liquid chromatography
- HPMC:
-
hypromellose
- IV:
-
intravenous
- MMAD:
-
mass median aerodynamic diameter
- MsLI:
-
multi-stage liquid impinger
- MTIC:
-
5-(3-methyltriazen-1-yl)imidazole-4-carboxamide
- NGI:
-
next generation impactor
- NSCLC:
-
non-small cell lung cancer
- P90H:
-
phospholipon 90H
- SCLC:
-
small cell lung cancer
- SEM:
-
scanning electron microscopy
- SLF:
-
simulated lung fluid
- TGA:
-
thermogravimetric analysis
- TMZ:
-
temozolomide
- XRPD:
-
X-ray powder diffraction
REFERENCES
Jemal A, Thun MJ, Ries LAG, Howe HL, Weir HK, Center MM, et al. Annual report to the nation on the status of cancer, 1975–2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst. 2008;100:1672–94.
Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–94.
Erhunmwunsee L, D’Amico T. Surgical management of pulmonary metastases. Ann Thorac Surg. 2009;88:2052–60.
Yano T, Shoji F, Maehara Y. Current status of pulmonary metastasectomy from primary epithelial tumours. Surg Today. 2009;39:91–7.
Smyth HDC, Saleem I, Donovan M, Verschraegen CF. Pulmonary delivery of anti-cancer agents. In: Williams RO, Taft DR, McConville JT, editors. Advanced drug formulation design to optimize therapeutic outcomes. New-York: Informa Healthcare; 2008. p. 81–111.
Sharma S, White D, Imondi AR, Placke ME, Vail DM, Kris MG. Development of inhalational agents for oncologic use. Am J Clin Oncol. 2001;19:1839–47.
Jain KK. Drug delivery systems—an overview. Methods Mol Biol. 2008;437:1–50.
Shevchenko IT, Resnik GE. Inhalation of chemical substances and oxygen in radiotherapy of bronchial cancer. Neoplasia. 1968;15:419–26.
Limper AH. Chemotherapy-induced lung disease. Clin Chest Med. 2004;25:53–64.
Gagnadoux F, Hureaux J, Vecellio L, Urban T, Le Pape A, Valo I, et al. Aerosolized chemotherapy. J Aerosol Med Pulm Drug Deliv. 2008;21:61–9.
Wittgen BPH, Kunst PWA, van der Born K, van Wijk AW, Perkins W, Pilkiewicz FG, et al. Phase I study of aerosolized SLIT cisplatine in the treatment of patients with carcinoma of the lung. Clin Cancer Res. 2007;13:2414–21.
Otterson GA, Villalona-Calero MA, Sharma S, Kris MG, Imondi A, Gerber M, et al. Phase I study of inhaled doxorubicin for patients with metastatic tumors to the lungs. Clin Cancer Res. 2007;13:1246–52.
Verschraegen CF, Gilbert BE, Loyer E, Huaringa A, Walsh G, Newman RA, et al. Clinical evaluation of the delivery and safety of aerosolized liposomal 9-nitro-20(S)-Camptothecin in patients with advanced pulmonary malignancies. Clin Cancer Res. 2004;10:2319–26.
O’Callaghan C, Barry PW. The science of nebulised drug delivery. Thorax. 1997;52:S31–44.
Pilcer G, Amighi K. Formulation strategy and use of excipients in pulmonary drug delivery. Int J Pharm. 2010;392:1–19.
Kleinstreuer C, Zhang Z. Targeted drug aerosol deposition analysis for a four-generation lung airway model with hemispherical tumors. J Biomech Eng. 2003;125:197–206.
Cancellieri A, Dalpiaz G, Maffessanti M, Pesci A, Polverosi R, Zompatori M. Diffuse Lung Diseases, clinical features, pathology, HRCT. New York: Springer; 2006.
Lefranc F, Facchini V, Kiss R. Proautophagic drugs: a novel means to combat apoptosis-resistant cancers, with a special emphasis on glioblastomas. Oncologist. 2007;12:1395–403.
Trinh VA, Pastel SP, Hwu WJ. The safety of temozolomide in the treatment of malignancies. Expert Opin Drug Saf. 2009;8:493–9.
Maldonado F, Limper AH, Kaiser KL, Aubry M-C. Temozolomide-associated organizing pneumonitis. Mayo Clin Proc. 2007;82:771–3.
Adonizio CS, Babb JS, Maiale C, Huang C, Donahue J, Millenson MM, et al. Temozolomide in non-small-cell lung cancer: preliminary results of a phase II trial in previously treated patients. Clin Lung Cancer. 2002;3:254–8.
Choong NW, Mauer AM, Hoffman PC, Rudin CM, Winegarden JD, Villano JL, et al. Phase II trial of temozolomide and irinotecan as second line treatment for advanced non-small cell lung cancer. J Thorac Oncol. 2006;1:732–3.
Kouroussis C, Vamvakas L, Vardakis N, Ktsakis A, Kalbakis K, Saridakis Z, et al. Continuous administration of daily low-dose temozolomide in pretreated patients with advanced non-small cell lung cancer: a phase II study. Oncology. 2009;76:112–7.
Lamoral-Theys D, Pottier L, Kerff F, Dufrasne F, Proutière F, Wauthoz N, et al. Simple di- and trivanillates exibit cytostatic properties towards cancer cells resistant to pro-apoptotic stimuli. Bioorg Med Chem. 2010;18:3823–33.
Wauthoz N, Deleuze P, Hecq J, Roland I, Saussez S, Adanja I, et al. In vivo assessment of témozolomide local delivery for lung cancer inhalation therapy. Eur J Pharm Sci. 2010;29:402–11.
Mathieu V, Le Mercier M, De Neve N, Sauvage S, Gras T, Roland I, et al. Galectin-1 knockdown increases sensitivity of temozlomide in a B16F10 mouse metastatic melanoma model. J Invest Dermatol. 2007;127:2399–410.
Sdraulig S, Franich R, Tinker RA, Solomon S, O’Brien R, Johnston PN. In vitro dissolution studies of uranium bearing material in simulated lung fluid. J Environ Radioact. 2008;99:527–38.
Shah VP, Tsong Y, Sathe P, Liu J-P. In vitro dissolution profile comparison-statistics and analysis of the similarity factor f2. Pharm Res. 1998;15:889–96.
Stupp R, Warren PM, van den Bent MJ, Weller M, Fisher B, Taphoorn MJB, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastomas. N Engl J Med. 2005;352:987–96.
Hecq J, Deleers M, Fanara D, Vrancks H, Amighi K. Preparation and characterization of nanocrystals for solubility and dissolution rate enhancement of nifedipine. Int J Pharm. 2005;299:167–77.
Pilcer G, Vanderbist F, Amighi K. Preparation and characterization of spray-dried tobramycin powders containing nanoparticles for pulmonary delivery. Int J Pharm. 2009;365:162–9.
Miao S, Ross YH. Crystallization kinetics and X-ray diffraction of crystals formed in amorphous lactose, threhalose, and lactose/trehalose mixtures. J Food Sci. 2005;70:350–8.
Pilcer G, Sebti T, Amighi K. Formulation and characterization of lipid-coated tobramycin particles for dry powder inhalation. Pharm Res. 2006;23:931–40.
Sebti T. Developpement et evaluation de formulations lipidiques à poudre sèche pour inhalation, Thèse (ULB, Pharmacie). Bruxelles: Université Libre de Bruxelles; 2006.
Drapier-Beche N, Fanni J, Parmentier M, Vilasi M. Evaluation of lactose crystalline forms by nondestructive analysis. J Dairy Sci. 1997;80:457–63.
Sung JC, Pulliam BL, Edwards DA. Nanoparticles for drug delivery to the lungs. Trends Biotechnol. 2007;25:563–70.
Davies NM, Feddah MR. A novel method for assessing dissolution of aerosol inhaler products. Int J Pharm. 2003;255:175–87.
Henning A, Schneider M, Nafee N, Muijs L, Rytting E, Wang X, et al. Influence of particle size and material properties on mucociliary clearance from the airways. J Aerosol Med Pulm Drug Deliv. 2010;23:233–41.
Yang W, Tam J, Miller DA, Zhou J, McConville JT, Johnston KP, et al. High bioavailability from nebulized itraconazole nanoparticle dispersions with biocompatible stabilizers. Int J Pharm. 2008;361:177–88.
Sampson JH, Archer GE, Villavicencio AT, McLendon RE, Friedman AH, Bishop WR, et al. Treatment of neoplastic meningitis with intrathecal temozolomide. Clin Cancer Res. 1999;5:1183–8.
Salama RO, Daniela T, Chan HK, Young PM. Preparation and characterisation of controlled release co-spray dried drug-polymer microparticles for inhalation 2: evaluation of in vitro release profiling methodologies for controlled release respiratory aerosols. Eur J Pharm Biopharm. 2008;70:145–52.
Son Y-J, McConville JT. Development of a standardized dissolution test method for inhaled pharmaceutical formulations. Int J Pharm. 2009;382:15–22.
ACKNOWLEDGMENTS
The authors would like to thank the Industrial Chemistry Department of ULB for the X-Ray Powder Diffraction. Robert Kiss is a Director of Research with the Fonds National de la Recherche Scientifique (FRS-FNRS, Brussels, Belgium).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wauthoz, N., Deleuze, P., Saumet, A. et al. Temozolomide-Based Dry Powder Formulations for Lung Tumor-Related Inhalation Treatment. Pharm Res 28, 762–775 (2011). https://doi.org/10.1007/s11095-010-0329-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0329-x