ABSTRACT
Purpose
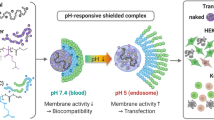
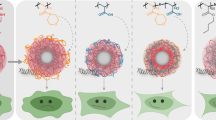
A reversibly-PEGylated diblock copolymer, poly(aspartate-hydrazide-poly(ethylene glycol))-block-poly(aspartate-diaminoethane) (p[Asp(Hyd-PEG)]-b-p[Asp(DET)]) was reported here for enhanced gene transfection and colloidal stability. The diblock copolymer possessed a unique architecture based on a poly(aspartamide) backbone. The first block, p[Asp(Hyd)], was used for multi-PEG conjugations, and the second block, p[Asp(DET)], was used for DNA condensation and endosomal escape.
Methods
p[Asp(Hyd-PEG)]-b-p[Asp(DET)] was synthesized and characterized by 1H-NMR. Polyplexes were formed by mixing the synthesized polymers and pDNA. The polyplex size, ζ-potential, and in vitro transfection efficiency were determined by dynamic light scattering, ζ-potential measurements, and luciferase assays, respectively. pH-dependent release of PEG from the polymer was monitored by cationic-exchange chromatography.
Results
The polyplexes were 70–90 nm in size, and the surface charge was effectively shielded by a PEG layer. The transfection efficiency of the reversibly PEGylated polyplexes was confirmed to be comparable to that of the non-PEGylated counterparts and 1,000 times higher than that of the irreversibly PEGylated polyplexes. PEG release was demonstrated to be pH-sensitive. Fifty percent of the PEG was released within 30 min at pH 5, while the polymer incubated at pH 7.4 could still maintain 50% of PEG after 8 h.
Conclusion
The reversibly PEGylated polyplexes were shown to maintain polyplex stability without compromising transfection efficiency.












Similar content being viewed by others
REFERENCES
Wiley. (2009) Gene Therapy Clinical Trials Worldwide. J Gene Med. http://www.wiley.co.uk/genetherapy/clinical/ (accessed 11/14/09).
Lehrman S. Virus treatment questioned after gene therapy death. Nature. 1999;401:517–8. doi:10.1038/43977.
Assessment of adenoviral vector safety and toxicity: report of the National Institutes of Health Recombinant DNA Advisory Committee. Hum Gene Ther. 13, 3–13 (2002) doi:10.1089/10430340152712629.
Cavazzana-Calvo M, Lagresle C, Hacein-Bey-Abina S, Fischer A. Gene therapy for severe combined immunodeficiency. Annu Rev Med. 2005;56:585–602. doi:10.1146/annurev.med.56.090203.104142.
Another case of gene therapy-associated leukemia. Biotechnology Law Report 27, 11 (2008).
Mastrobattista E, van der Aa MA, Hennink WE, Crommelin DJ. Artificial viruses: a nanotechnological approach to gene delivery. Nat Rev Drug Discov. 2006;5:115–21.
Meyer M, Wagner E. Recent developments in the application of plasmid DNA-based vectors and small interfering RNA therapeutics for cancer. Hum Gene Ther. 2006;17:1062–76. doi:10.1089/hum.2006.17.1062.
Merdan T, Kopecek J, Kissel T. Prospects for cationic polymers in gene and oligonucleotide therapy against cancer. Adv Drug Deliv Rev. 2002;54:715–58. doi:S0169409X02000467 [pii].
Park TG, Jeong JH, Kim SW. Current status of polymeric gene delivery systems. Adv Drug Deliv Rev. 2006;58:467–86. doi:S0169-409X(06)00050-0 [pii]10.1016/j.addr.2006.03.007.
Pack DW, Hoffman AS, Pun S, Stayton PS. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–93. doi:nrd1775 [pii]10.1038/nrd1775.
Pathak A, Patnaik S, Gupta KC. Recent trends in non-viral vector-mediated gene delivery. Biotechnol J. 2009;4:1559–72. doi:10.1002/biot.200900161.
Chonn A, Semple SC, Cullis PR. Association of blood proteins with large unilamellar liposomes in vivo. Relation to circulation lifetimes. J Biol Chem. 1992;267:18759–65.
Ogris M, Brunner S, Schuller S, Kircheis R, Wagner E. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 1999;6:595–605. doi:10.1038/sj.gt.3300900.
Petersen H, Fechner PM, Martin AL, Kunath K, Stolnik S, Roberts CJ, et al. Polyethylenimine-graft-poly(ethylene glycol) copolymers: influence of copolymer block structure on DNA complexation and biological activities as gene delivery system. Bioconjug Chem. 2002;13:845–54. doi:bc025529v [pii].
Mishra S, Webster P, Davis ME. PEGylation significantly affects cellular uptake and intracellular trafficking of non-viral gene delivery particles. Eur J Cell Biol. 2004;83:97–111.
Harada A, Cammas S, Kataoka K. Stabilized α-helix structure of poly(l-lysine)-block-poly(ethylene glycol) in aqueous medium through supramolecular assembly. Macromolecules. 1996;29:6183–8.
Hatakeyama H, Ito E, Akita H, Oishi M, Nagasaki Y, Futaki S, et al. A pH-sensitive fusogenic peptide facilitates endosomal escape and greatly enhances the gene silencing of siRNA-containing nanoparticles in vitro and in vivo. J Control Release. 2009;139:127–32. doi:S0168-3659(09)00418-0 [pii]10.1016/j.jconrel.2009.06.008.
O’Riordan CR, Lachapelle A, Delgado C, Parkes V, Wadsworth SC, Smith AE, et al. PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum Gene Ther. 1999;10:1349–58. doi:10.1089/10430349950018021.
Woodle MC. Controlling liposome blood clearance by surface-grafted polymers. Adv Drug Deliv Rev. 1998;32:139–52. doi:S0169-409X(97)00136-1 [pii].
Walker GF, Fella C, Pelisek J, Fahrmeir J, Boeckle S, Ogris M, et al. Toward synthetic viruses: endosomal pH-triggered deshielding of targeted polyplexes greatly enhances gene transfer in vitro and in vivo. Mol Ther. 2004;11:418–25.
Han M, Bae Y, Nishiyama N, Miyata K, Oba M, Kataoka K. Transfection study using multicellular tumor spheroids for screening non-viral polymeric gene vectors with low cytotoxicity and high transfection efficiencies. J Control Release. 2007;121:38–48. doi:S0168-3659(07)00238-6 [pii]10.1016/j.jconrel.2007.05.012.
Xiong MP, Bae Y, Fukushima S, Forrest ML, Nishiyama N, Kataoka K, et al. pH-responsive Multi-PEGylated dual cationic nanoparticles enable charge modulations for safe gene delivery. ChemMedChem. 2007;2:1321–7. doi:10.1002/cmdc.200700093.
Takae S, Miyata K, Oba M, Ishii T, Nishiyama N, Itaka K, et al. PEG-detachable polyplex micelles based on disulfide-linked block catiomers as bioresponsive nonviral gene vectors. J Am Chem Soc. 2008;130:6001–9. doi:10.1021/ja800336v.
Choi JS, MacKay JA, Szoka Jr FC. Low-pH-sensitive PEG-stabilized plasmid-lipid nanoparticles: preparation and characterization. Bioconjug Chem. 2003;14:420–9. doi:10.1021/bc025625w.
West KR, Otto S. Reversible covalent chemistry in drug delivery. Curr Drug Discov Technol. 2005;2:123–60.
Huang M, Ma Z, Khor E, Lim LY. Uptake of FITC-chitosan nanoparticles by A549 cells. Pharm Res. 2002;19:1488–94.
Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377:159–69. doi:10.1042/BJ20031253BJ20031253 [pii].
Kim HR, Gil S, Andrieux K, Nicolas V, Appel M, Chacun H, et al. Low-density lipoprotein receptor-mediated endocytosis of PEGylated nanoparticles in rat brain endothelial cells. Cell Mol Life Sci. 2007;64:356–64. doi:10.1007/s00018-007-6390-x.
Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis Physiol Rev. 1997;77:759–803.
Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev. 2007;59:748–58. doi:S0169-409X(07)00096-8 [pii]10.1016/j.addr.2007.06.008.
Remaut K, Lucas B, Braeckmans K, Demeester J, De Smedt SC. Pegylation of liposomes favours the endosomal degradation of the delivered phosphodiester oligonucleotides. J Control Release. 2007;117:256–66. doi:S0168-3659(06)00589-X [pii]10.1016/j.jconrel.2006.10.029.
Miyata K, Oba M, Nakanishi M, Fukushima S, Yamasaki Y, Koyama H, et al. Polyplexes from poly(aspartamide) bearing 1, 2-diaminoethane side chains induce pH-selective, endosomal membrane destabilization with amplified transfection and negligible cytotoxicity. J Am Chem Soc. 2008;130:16287–94. doi:10.1021/ja804561g.
Harada-Shiba M, Takamisawa I, Miyata K, Ishii T, Nishiyama N, Itaka K, et al. Intratracheal gene transfer of adrenomedullin using polyplex nanomicelles attenuates monocrotaline-induced pulmonary hypertension in rats. Mol Ther. 2009;17:1180–6. doi:mt200963 [pii]10.1038/mt.2009.63.
Darly WH, Poche D. The preparation of N-carboxyanhydrides of alphaaminoacids using bis(trichloromethyl)carbonate. Tetrahedron Lett. 1988;29:5859–62.
Skoog B. Determination of polyethylene glycols 4000 and 6000 in plasma protein preparations. Vox sanguinis. 1979;37:345–9. doi:10.1111/j.1423-0410.1979.tb02314.x.
Pun SH, Davis ME. Development of a nonviral gene delivery vehicle for systemic application. Bioconjug Chem. 2002;13:630–9. doi:bc0155768 [pii].
Meyer M, Philipp A, Oskuee R, Schmidt C, Wagner E. Breathing life into polycations: functionalization with pH-responsive endosomolytic peptides and polyethylene glycol enables siRNA delivery. J Am Chem Soc. 2008;130:3272–3. doi:10.1021/ja710344v.
Wolfert MA, Schacht EH, Toncheva V, Ulbrich K, Nazarova O, Seymour LW. Characterization of vectors for gene therapy formed by self-assembly of DNA with synthetic block co-polymers. Hum Gene Ther. 1996;7:2123–33. doi:10.1089/hum.1996.7.17-2123.
Gref R, Luck M, Quellec P, Marchand M, Dellacherie E, Harnisch S, et al. ‘Stealth’ corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B: Biointerfaces. 2000;18:301–13. doi:S0927-7765(99)00156-3 [pii].
Miyata K, Fukushima S, Nishiyama N, Yamasaki Y, Kataoka K. PEG-based block catiomers possessing DNA anchoring and endosomal escaping functions to form polyplex micelles with improved stability and high transfection efficacy. J Control Release. 2007;122:252–60. doi:S0168-3659(07)00303-3 [pii]10.1016/j.jconrel.2007.06.020.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lai, T.C., Bae, Y., Yoshida, T. et al. pH-Sensitive Multi-PEGylated Block Copolymer as a Bioresponsive pDNA Delivery Vector. Pharm Res 27, 2260–2273 (2010). https://doi.org/10.1007/s11095-010-0092-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-010-0092-z