Abstract
Purpose
To determine the transscleral permeability of chemotherapeutic drugs vinblastine and doxorubicin for treatment of intraocular tumors, and to compare the use of doxorubicin encapsulated in PLGA and liposome nanoparticles.
Methods
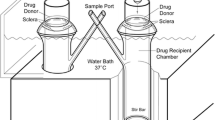
Human sclera was isolated and mounted in a Lucite chamber. Fluorescently tagged vinblastine (VIN), innately fluorescent free doxorubicin (DOX), PLGA doxorubicin (PLGA-DOX), or Doxil (Tibotec Therapeutics) were added to the episcleral donor chamber. The choroidal side was perfused with Balanced Salt Solution. Perfusate fractions were collected over 24 h and measured for fluorescence. Following the experiment, tissue sections were imaged, underwent a drug wash out procedure, and tissue drug content was analyzed using an LC–MS/MS method.
Results
Within 24 h, a total of 68%, 74%, 29%, and 1.9% of the drug dose from VIN, DOX, PLGA-DOX, and Doxil, respectively, diffused across the sclera. VIN and DOX scleral tissue showed strong fluorescence after 24 h. PLGA-DOX displayed scattered fluorescence, and Doxil indicated minimal fluorescence. LC–MS/MS revealed strong tissue binding of DOX.
Conclusions
This study suggests both vinblastine and doxorubicin are able to diffuse across human sclera. In addition, PLGA nanoparticles delivered doxorubicin at a slower rate across the sclera, and the liposome preparation resulted in the slowest delivery of drug.




Similar content being viewed by others
REFERENCES
A. L. Murphree, J. G. Villablanca, W. F. Deegan 3rd., J. K. Sato, M. Malogolowkin, A. Fisher, R. Parker, E. Reed, and C. J. Gomer. Chemotherapy plus local treatment in the management of intraocular retinoblastoma. Arch. Ophthalmol. 114:1348–1356 (1996).
M. S. Benz, I. U. Scott, T. G. Murray, D. Kramer, and S. Toledano. Complications of systemic chemotherapy as treatment of retinoblastoma. Arch. Ophthalmol. 118:577–578 (2000).
B. L. Gallie, A. Budning, G. DeBoer, J. J. Thiessen, G. Koren, Z. Verjee, V. Ling, and H. S. Chan. Chemotherapy with focal therapy can cure intraocular retinoblastoma without radiotherapy. Arch. Ophthalmol. 114:1321–1328 (1996).
M. Chintagumpala, P. Chevez-Barrios, E. A. Paysse, S. E. Plon, and R. Hurwitz. Retinoblastoma: review of current management. Oncologist. 12:1237–1246 (2007). doi:10.1634/theoncologist.12-10-1237.
P. De Potter. Current treatment of retinoblastoma. Curr. Opin. Ophthalmol. 13:331–336 (2002). doi:10.1097/00055735-200210000-00007.
D. H. Abramson, C. M. Frank, and I. J. Dunkel. A phase I/II study of subconjunctival carboplatin for intraocular retinoblastoma. Ophthalmology. 106:1947–1950 (1999). doi:10.1016/S0161-6420(99)90406-2.
L. Lenazand, and J. A. Page. Cardiotoxicity of adriamycin and related anthracyclines. Cancer Treat. Rev. 3:111–120 (1976). doi:10.1016/S0305-7372(76)80018-7.
K. R. Van Quill, P. K. Dioguardi, C. T. Tong, J. A. Gilbert, T. M. Aaberg Jr., H. E. Grossniklaus, H. F. Edelhauser, and J. M. O’Brien. Subconjunctival carboplatin in fibrin sealant in the treatment of transgenic murine retinoblastoma. Ophthalmology. 112:1151–1158 (2005). doi:10.1016/j.ophtha.2004.11.060.
T. G. Murray, N. Cicciarelli, J. M. O’Brien, E. Hernandez, R. L. Mueller, B. J. Smith, and W. Feuer. Subconjunctival carboplatin therapy and cryotherapy in the treatment of transgenic murine retinoblastoma. Arch. Ophthalmol. 115:1286–1290 (1997).
A. E. Simpson, J. A. Gilbert, D. E. Rudnick, D. H. Geroski, T. M. Aaberg Jr., and H. F. Edelhauser. Transscleral diffusion of carboplatin: an in vitro and in vivo study. Arch. Ophthalmol. 120:1069–1074 (2002).
A. Makimoto. Results of treatment of retinoblastoma that has infiltrated the optic nerve, is recurrent, or has metastasized outside the eyeball. Int. J. Clin. Oncol. 9:7–12 (2004). doi:10.1007/s10147-003-0364-2.
F. Di Nicolantonio, M. Neale, Z. Onadim, J. L. Hungerford, J. L. Kingston, and I. A. Cree. The chemosensitivity profile of retinoblastoma. Recent Results Cancer Res. 161:73–80 (2003).
Physicians’ Desk Reference, Vol. 55th ed, Medical Economics Company, Inc., Montvale, 2001, pp. 1986–2565.
R. Dhamodharan, M. A. Jordan, D. Thrower, L. Wilson, and P. Wadsworth. Vinblastine suppresses dynamics of individual microtubules in living interphase cells. Mol. Biol. Cell. 6:1215–1229 (1995).
T. Hu, Q. Le, Z. Wu, and W. Wu. Determination of doxorubicin in rabbit ocular tissues and pharmacokinetics after intravitreal injection of a single dose of doxorubicin-loaded poly-beta-hydroxybutyrate microspheres. J. Pharm. Biomed. Anal. 43:263–269 (2007). doi:10.1016/j.jpba.2006.06.032.
T. W. Olsen, H. F. Edelhauser, J. I. Lim, and D. H. Geroski. Human scleral permeability: effects of age, cryotherapy, transscleral diode laser, and surgical thinning. Investig. Ophthalmol. Vis. Sci. 36:1893–1903 (1995).
D. E. Rudnick, J. S. Noonan, D. H. Geroski, M. R. Prausnitz, and H. F. Edelhauser. The effect of intraocular pressure on human and rabbit scleral permeability. Invest. Ophthalmol. Vis. Sci. 40:3054–3058 (1999).
J. A. Gilbert, A. E. Simpson, D. E. Rudnick, D. H. Geroski, T. M. Aaberg Jr., and H. F. Edelhauser. Transscleral permeability and intraocular concentrations of cisplatin from a collagen matrix. J. Control. Release. 89:409–417 (2003). doi:10.1016/S0168-3659(03)00151-2.
A. C. Amriteand, and U. B. Kompella. Size-dependent disposition of nanoparticles and microparticles following subconjunctival administration. J. Pharm. Pharmacol. 57:1555–1563 (2005). doi:10.1211/jpp.57.12.0005.
A. C. Amrite, H. F. Edelhauser, S. R. Singh, and U. B. Kompella. Effect of circulation on the disposition and ocular tissue distribution of 20 nm nanoparticles after periocular administration. Mol. Vis. 14:150–160 (2008).
J. Ambati, C. S. Canakis, J. W. Miller, E. S. Gragoudas, A. Edwards, D. J. Weissgold, I. Kim, F. C. Delori, and A. P. Adamis. Diffusion of high molecular weight compounds through sclera. Invest. Ophthalmol. Vis. Sci. 41:1181–1185 (2000).
L. P. Cruysberg, R. M. Nuijts, D. H. Geroski, L. H. Koole, F. Hendrikse, and H. F. Edelhauser. In vitro human scleral permeability of fluorescein, dexamethasone-fluorescein, methotrexate-fluorescein and rhodamine 6G and the use of a coated coil as a new drug delivery system. J. Ocul. Pharmacol. Ther. 18:559–569 (2002). doi:10.1089/108076802321021108.
U. B. Kompella, N. Bandi, and S. P. Ayalasomayajula. Subconjunctival nano- and microparticles sustain retinal delivery of budesonide, a corticosteroid capable of inhibiting VEGF expression. Invest. Ophthalmol. Vis. Sci. 44:1192–1201 (2003). doi:10.1167/iovs.02-0791.
S. P. Ayalasomayajulaand, and U. B. Kompella. Retinal delivery of celecoxib is several-fold higher following subconjunctival administration compared to systemic administration. Pharm. Res. 21:1797–1804 (2004). doi:10.1023/B:PHAM.0000045231.51924.e8.
M. R. Robinson, S. S. Lee, H. Kim, S. Kim, R. J. Lutz, C. Galban, P. M. Bungay, P. Yuan, N. S. Wang, J. Kim, and K. G. Csaky. A rabbit model for assessing the ocular barriers to the transscleral delivery of triamcinolone acetonide. Exp. Eye Res. 82:479–487 (2006). doi:10.1016/j.exer.2005.08.007.
ACKNOWLEDGMENTS
This research was supported by grants from Fight for Sight, Research to Prevent Blindness, and NEI Grants: P30 EY06360 and R24 EY017045.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, E.S., Durairaj, C., Kadam, R.S. et al. Human Scleral Diffusion of Anticancer Drugs from Solution and Nanoparticle Formulation. Pharm Res 26, 1155–1161 (2009). https://doi.org/10.1007/s11095-009-9835-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-009-9835-0