Abstract
Purpose
To better understand the importance of the environmental conditions for drug release from biodegradable microparticles allowing for the development of more appropriate in vitro release measurement techniques.
Methods
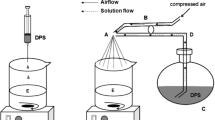
Propranolol HCl diffusion in various agarose gels was characterized by NMR and UV analysis. Fick’s law was used to theoretically predict the mass transport kinetics. Drug release from PLGA-based microparticles in such agarose gels was compared to that measured in agitated bulk fluids (“standard” method).
Results
NMR analysis revealed that the drug diffusivity was almost independent of the hydrogel concentration, despite of the significant differences in the systems’ mechanical properties. This is due to the small size of the drug molecules/ions with respect to the hydrogel mesh size. Interestingly, the theoretically predicted drug concentration-distance-profiles could be confirmed by independent experiments. Most important from a practical point of view, significant differences in the release rates from the same batch of PLGA-based microparticles into a well agitated bulk fluid versus a semi-solid agarose gel were observed.
Conclusion
Great care must be taken when defining the in vitro conditions for drug release measurements from biodegradable microparticles. The obtained new insight can help facilitating the development of more appropriate in vitro release testing procedures.








Similar content being viewed by others
References
P. B. O’Donnell, and J.W. McGinity. Preparation of microspheres by the solvent evaporation technique. Adv. Drug Deliv. Rev. 28:25–42 (1997). doi:10.1016/S0169-409X(97)00049-5.
S. Freiberg, and X. X. Zhu. Polymer microspheres for controlled drug release. Int. J. Pharm. 282:1–18 (2004). doi:10.1016/j.ijpharm.2004.04.013.
G. E. Visscher, R. L. Robison, H. V. Maulding, J. W. Fong, J. E. Pearson, and G. J. Argentieri. Biodegradation of and tissue reaction to 50:50 poly(dl-lactide-co-glycolide) microcapsules. J. Biomed. Mater. Res. 19:349–365 (1985). doi:10.1002/jbm.820190315.
J. M. Anderson, and M. S. Shive. Biodegradation and biocompatibility of PLA and PLGA microspheres. Adv. Drug Deliv. Rev. 28:5–24 (1997). doi:10.1016/S0169-409X(97)00048-3.
J. C. Middleton, and A. J. Tipton. Synthetic biodegradable polymers as orthopedic devices. Biomaterials. 21:2335–2346 (2000). doi:10.1016/S0142-9612(00)00101-0.
L. Wu, and J. Ding. In vitro degradation of three-dimensional porous poly(d,l-lactide-co-glycolide) scaffolds for tissue engineering. Biomaterials. 25:5821–5830 (2004). doi:10.1016/j.biomaterials.2004.01.038.
E. Fournier, C. Passirani, C. N. Montero-Menei, and J. P. Benoit. Biocompatibility of implantable synthetic polymeric drug carriers: focus on brain biocompatibility. Biomaterials. 24:3311–3331 (2003). doi:10.1016/S0142-9612(03)00161-3.
P. Menei, E. Jadaud, N. Faisant, M. Boisdron-Celle, S. Michalak, D. Fournier, M. Delhaye, and J. P. Benoit. Stereotaxic implantation of 5-Fluorouracil-releasing microspheres in malignant glioma. Cancer. 100:405–410 (2004). doi:10.1002/cncr.11922.
D. J. Burgess, A. S. Hussain, T. S. Ingallinera, and M. L. Chen. Assuring quality and performance of sustained and controlled release parenterals: AAPS workshop report co-sponsored by FDA and USP. Pharm. Res. 19:1761–1768 (2002). doi:10.1023/A:1020730102176.
D. Burgess, D. Crommelin, A. Hussain, and M. Chen. Assuring quality and performance of sustained and controlled release parenterals. Eur. J. Pharm. Sci. 21:679–690 (2004). doi:10.1016/j.ejps.2004.03.001.
C. Nastruzzi, E. Esposito, R. Cortesi, R. Gambari, and E. Menegatti. Kinetics of bromocriptine release from microspheres: comparative analysis between different in vitro models. J. Microencapsul. 11:565–574 (1993). doi:10.3109/02652049409034995.
B. Conti, I. Genta, P. Giunchedi, and T. Modena. Testing of “in vitro” dissolution behaviour of microparticulate drug delivery systems. Drug Dev. Ind. Pharm. 21:1223–1233 (1995). doi:10.3109/03639049509026671.
D. F. Bain, D. L. Munday, and A. Smith. Modulation of rifampicin release from spray-dried microspheres using combinations of poly-(d,l-lactide). J. Microencapsul. 16:369–385 (1999). doi:10.1080/026520499289086.
A. Aubert-Pouëssel, D. C. Bibby, M. C. Venier-Julienne, F. Hindré, and J. P. Benoit. A novel in vitro delivery system for assessing the biological integrity of protein upon release from PLGA microspheres. Pharm. Res. 19:1046–1051 (2002). doi:10.1023/A:1016482809810.
S. D’Souza, and P. P. DeLuca. Development of a dialysis in vitro release method for biodegradable microspheres. AAPS PharmSciTech. 6:323–328 (2005). doi:10.1208/pt060242.
S. D’Souza, and P. P. DeLuca. Methods to assess in vitro drug release from injectable polymeric particulate systems. Pharm. Res. 23:460–474 (2006). doi:10.1007/s11095-005-9397-8.
J. Siepmann, and A. Goepferich. Mathematical modeling of bioerodible, polymeric drug delivery systems. Adv. Drug Deliv. Rev. 48:229–247 (2001). doi:10.1016/S0169-409X(01)00116-8.
A. Brunner, K. Maeder, and A. Goepferich. PH and osmotic pressure inside biodegradable microspheres during erosion. Pharm. Res. 16:847–853 (1999). doi:10.1023/A:1018822002353.
K. Fu, D. W. Pack, A. M. Klibanov, and R. Langer. Visual evidence of acidic environment within degrading poly(lactic-co-glycolic acid) (PLGA) microspheres. Pharm. Res. 17:100–106 (2000). doi:10.1023/A:1007582911958.
L. Li, and S. P. Schwendeman. Mapping neutral microclimate pH in PLGA microspheres. J. Control. Release. 101:163–173 (2005). doi:10.1016/j.jconrel.2004.07.029.
F. v. Burkersroda, L. Schedl, and A. Goepferich. Why degradable polymers undergo surface erosion or bulk erosion. Biomaterials. 23:4221–4231 (2002). doi:10.1016/S0142-9612(02)00170-9.
S. P. Schwendeman. Recent advances in the stabilization of proteins encapsulated in injectable PLGA delivery systems. Crit. Rev. Ther. Drug Carrier Syst. 19:73–98 (2002). doi:10.1615/CritRevTherDrugCarrierSyst.v19.i1.20.
J. Siepmann, K. Elkharraz, F. Siepmann, and D. Klose. How autocatalysis accelerates drug release from PLGA-based microparticles: A quantitative treatment. Biomacromolecules. 6:2312–2319 (2005). doi:10.1021/bm050228k.
D. Klose, F. Siepmann, K. Elkharraz, S. Krenzlin, and J. Siepmann. How porosity and size affect the drug release mechanisms from PLGA-based microparticles. Int. J. Pharm. 314:198–206 (2006). doi:10.1016/j.ijpharm.2005.07.031.
J. Siepmann, F. Siepmann, and A. T. Florence. Local controlled drug delivery to the brain: Mathematical modeling of the underlying mass transport mechanisms. Int. J. Pharm. 314:101–119 (2006). doi:10.1016/j.ijpharm.2005.07.027.
S. Allababidi, and J. C. Shah. Kinetics and mechanism of release from glyceryl monostearate-based implants: evaluation of release in a gel simulating in vivo implantation. J. Pharm. Sci. 87:738–744 (1998). doi:10.1021/js9703986.
G. T. Gillies, T. D. Wilhelm, J. A. C. Humphrey, H. L. Fillmore, K. L. Holloway, and W. C. Broaddus. A spinal cord surrogate with nanoscale porosity for in vitro simulations of restorative neurosurgical techniques. Nanotechnology. 13:587–591 (2002). doi:10.1088/0957-4484/13/5/308.
D. L. Holligan, G. T. Gillies, and J. P. Dailey. Magnetic guidance of ferrofluidic nanoparticles in an in vitro model of intraocular retinal repair. Nanotechnology. 14:661–666 (2003). doi:10.1088/0957-4484/14/6/318.
Z. Chen, G. Gillies, W. Broaddus, S. Prabhu, H. Fillmore, R. Mitchell, F. Corwin, and P. Fatouros. A realistic brain tissue phantom for intraparenchymal infusion studies. J. Neurosurg. 101:314–322 (2004).
A. S. Hoffman. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 54:3–12 (2002). doi:10.1016/S0169-409X(01)00239-3.
S. Arnott, A. Fulmer, W. E. Scott, I. C. Dea, R. Moorhouse, and D. A. Rees. The agarose double helix and its function in agarose gel structure. J. Mol. Biol. 90:269–272 (1974). doi:10.1016/0022-2836(74)90372-6.
M. Maaloum, N. Pernodet, and B. Tinland. Agarose gel structure using atomic force microscopy: Gel concentration and ionic strength effects. Electrophoresis. 19:1606–1610 (1998). doi:10.1002/elps.1150191015.
A. Pluen, P. A. Netti, R. K. Jain, and D. A. Berk. Diffusion of macromolecules in agarose gels: Comparison of linear and globular configurations. Biophys. J. 77:542–552 (1999).
J. Crank. The mathematics of diffusion. Clarendon Press, Oxford, 1975.
H. Sjoeberg, S. Persson, and N. Caram-Lelham. How interactions between drugs and agarose-carrageenan hydrogels influence the simultaneous transport of drugs. J. Control. Release. 59:391–400 (1999). doi:10.1016/S0168-3659(99)00013-9.
G. Spenlehauer, M. Vert, J. P. Benoit, and A. Boddaert. In vitro and in vivo degradation of poly(D,L lactide/glycolide) type microspheres made by solvent evaporation method. Biomaterials. 10:557–563 (1989). doi:10.1016/0142-9612(89)90063-X.
P. Menei, V. Daniel, C. Montero-Menei, M. Brouillard, A. Pouplard- Barthelaix, and J. P. Benoit. Biodegradation and brain tissue reaction to poly(d,l-lactide-co-glycolide) microspheres. Biomaterials. 14:470–478 (1993). doi:10.1016/0142-9612(93)90151-Q.
M. A. Tracy, K. L. Ward, L. Firouzabadian, Y. Wang, N. Dong, R. Qian, and Y. Zhang. Factors affecting the degradation rate of poly(lactide-co-glycolide) microspheres in vivo and in vitro. Biomaterials. 20:1057–1062 (1999). doi:10.1016/S0142-9612(99)00002-2.
B. H. Woo, J. W. Kostanski, S. Gebrekidan, B. A. Dani, B. C. Thanoo, and P. P. DeLuca. Preparation, characterization and in vivo evaluation of 120-day poly(D,L-lactide) leuprolide microspheres. J. Control. Release. 75:307–315 (2001). doi:10.1016/S0168-3659(01)004030-5.
M. Sandor, J. Harris, and E. Mathiowitz. A novel polyethylene depot device for the study of PLGA and P(FASA) microspheres in vitro and in vivo. Biomaterials. 23:4413–4423 (2002). doi:10.1016/S0142-9612(02)00183-7.
L. Lu, S. J. Peter, M. D. Lyman, H. L. Lai, S. M. Leite, J. A. Tamada, S. Uyama, J. P. Vacanti, R. Langer, and A. G. Mikos. In vitro and in vivo degradation of porous poly(-lactic-co-glycolic acid) foams. Biomaterials. 21:1837–1845 (2000). doi:10.1016/S0142-9612(00)00047-8.
R. A. Kenley, M. O. Lee, T. R. Mahoney, and L. M. Sanders. Poly(lactide-co-glycolide) decomposition kinetics in vivo and in vitro. Macromolecules. 20:2398–2403 (1987). doi:10.1021/ma00176a012.
M. Baro, E. Sanchez, A. Delgado, A. Perera, and C. Evora. In vitro-in vivo characterization of gentamicin bone implants. J. Control. Release. 83:353–364 (2002). doi:10.1016/S0168-3659(02)00179-7.
Acknowledgements
The authors are grateful for the support of this work by the French Association for Cancer Research “ARC” (“Association pour la Recherche sur le Cancer”: postdoctoral fellowship for Dr. Florence Siepmann and doctoral fellowship for Mrs. Diana Klose). The NMR facilities were funded by the “Nord-Pas de Calais” Regional Council, the French Ministry and European Regional Development Fonds (FEDER).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klose, D., Azaroual, N., Siepmann, F. et al. Towards More Realistic In Vitro Release Measurement Techniques for Biodegradable Microparticles. Pharm Res 26, 691–699 (2009). https://doi.org/10.1007/s11095-008-9747-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9747-4