Abstract
Purpose
The amorphous form of a drug may provide enhanced solubility, dissolution rate, and bioavailability but will also potentially crystallize over time. Miscible polymeric additives provide a means to increase physical stability. Understanding the miscibility of drug–polymer systems is of interest to optimize the formulation of such systems. The purpose of this work was to develop experimental models which allow for more quantitative estimates of the thermodynamics of mixing amorphous drugs with glassy polymers.
Materials and Methods
The thermodynamics of mixing several amorphous drugs with amorphous polymers was estimated by coupling solution theory with experimental data. The entropy of mixing was estimated using Flory–Huggins lattice theory. The enthalpy of mixing and any deviations from the entropy as predicted by Flory–Huggins lattice theory were estimated using two separate experimental techniques; (1) melting point depression of the crystalline drug in the presence of the amorphous polymer was measured using differential scanning calorimetry and (2) determination of the solubility of the drug in 1-ethyl-2-pyrrolidone. The estimated activity coefficient was used to calculate the free energy of mixing of the drugs in the polymers and the corresponding solubility.
Results
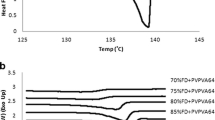
Mixtures previously reported as miscible showed various degrees of melting point depression while systems reported as immiscible or partially miscible showed little or no melting point depression. The solubility of several compounds in 1-ethyl-2-pyrrolidone predicts that most drugs have a rather low solubility in poly(vinylpyrrolidone).
Conclusions
Miscibility of various drugs with polymers can be explored by coupling solution theories with experimental data. These approximations provide insight into the physical stability of drug–polymer mixtures and the thermodynamic driving force for crystallization.






Similar content being viewed by others
References
D. Gulsen, C.C. Li, and A. Chauhan. Dispersion of DMPC liposomes in contact lenses for ophthalmic drug delivery. Curr. Eye Res. 30:1071–1080 (2005). doi:10.1080/02713680500346633.
I.P. Kaur, and M. Kanwar. Ocular preparations: The formulation approach. Drug. Dev. Ind. Pharm. 28:473–493 (2002). doi:10.1081/DDC-120003445.
M. Diestelhorst, and G.K. Krieglstein. The ocular tolerability of a new ophthalmic drug-delivery system (NODS). Int. Ophthalmol. 18:1–4 (1994). doi:10.1007/BF00919405.
G.A. Lesher, and G.G. Gunderson. Continuous drug-delivery through the use of disposable contact-lenses. Optom. Vis. Sci. 70:1012–1018 (1993). doi:10.1097/00006324-199312000-00004.
M.V. Zelenskaya, E.F. Baru, G.A. Babich, A.A. Kivaev, A.A. Suprun, and A.A. Ryabtseva. Soft contact-lenses impregnated with hypotensive drugs in the treatment of glaucoma. Vestn. Oftalmol. 102(3):14–17 (1986).
V.J. Marmion, and M.R. Jain. Role of soft contact-lenses and delivery of drugs. Trans. Ophthalmol. Soc. U. K. 96:319–321 (1976).
S.R. Waltman and H.E. Kaufman. Use of hydrophilic contact lenses to increase ocular penetration of topical drugs. Invest. Ophthalmol. 9:250–255 (1970).
B. Tesfamariam. Drug release kinetics from stent device-based delivery systems. Invest. Ophthalmol. 51:118–125 (2008).
J.P. Chen. Safety and efficacy of the drug-eluting stent: A double-edged sword? South Med. J. 101:174–178 (2008).
N. Kukreja, Y. Onuma, J. Daemen, and P.W. Serruys. The future of drug-eluting stents. Pharmacol. Res. 57:171–180 (2008). doi:10.1016/j.phrs.2008.01.012.
J.L. Ifkovits, and J.A. Burdick. Review: Photopolymerizable and degradable biomaterials for tissue engineering applications. Tissue Eng. 13:2369–2385 (2007). doi:10.1089/ten.2007.0093.
W.K. Wan, L. Yang, and D.T. Padavan. Use of degradable and nondegradable nanomaterials for controlled release. Nanomedicine. 2:483–509 (2007). doi:10.2217/17435889.2.4.483.
T. Sharkawi, F. Cornhill, A. Lafont, P. Sabaria, and M. Vert. Intravascular bioresorbable polymeric stents: A potential alternative to current drug eluting metal stents. J. Pharm. Sci. 96:2829–2837 (2007). doi:10.1002/jps.20957.
C.B. Packhaeuser, J. Schnieders, C.G. Oster, and T. Kissel. In situ forming parenteral drug delivery systems: An overview. Eur. J. Pharm. Biopharm. 58:445–455 (2004). doi:10.1016/j.ejpb.2004.03.003.
C. Vauthier, C. Dubernet, E. Fattal, H. Pinto-Alphandary, and P. Couvreur. Poly(alkylcyanoacrylates) as biodegradable materials for biomedical applications. Adv. Drug Deliv. Rev. 55:519–548 (2003). doi:10.1016/S0169-409X(03)00041-3.
H. Kimura, and Y. Ogura. Biodegradable polymers for ocular drug delivery. Ophthalmologica. 215:143–155 (2001). doi:10.1159/000050849.
J. Heller. Biodegradable polymers in controlled drug delivery. Crit. Rev. Ther. Drug Carrier Syst. 1:39–90 (1984).
R.G. Buckles. Biomaterials for drug delivery systems. J. Biomed. Materi. Res. 17:109–128 (1983). doi:10.1002/jbm.820170110.
W. Schneider, W.D. Bussmann, A. Hartmann, and M. Kaltenbach. Nitrate therapy in heart-failure. Cardiology. 79:5–13 (1991).
P.A. Todd, K.L. Goa, and H.D. Langtry. Transdermal nitroglycerin (glyceryl trinitrate)—A review of its pharmacology and therapeutic use. Drugs. 40:880–902 (1990). doi:10.2165/00003495-199040060-00009.
J.L. Ford. The current status of solid dispersions. Pharm. Acta Helv. 61:69–88 (1986).
A.T.M. Serajuddin. Solid dispersion of poorly water-soluble drugs: Early promises, subsequent problems, and recent breakthroughs. J. Pharm. Sci. 88:1058–1066 (1999). doi:10.1021/js980403l.
S. Sethia, and E. Squillante. Solid dispersions: Revival with greater possibilities and applications in oral drug delivery. Crit. Rev. Ther. Drug Carr. Syst. 20:215–247 (2003). doi:10.1615/CritRevTherDrugCarrierSyst.v20.i23.40.
V. Mummaneni, and R.C. Vasavada. Solubilization and dissolution of famotidine from solid glass dispersions of xylitol. Int. J. Pharm. 66:71–77 (1990). doi:10.1016/0378-5173(90)90386-I.
D.Q.M. Craig. The mechanisms of drug release from solid dispersions in water-soluble polymers. Int. J. Pharm. 231:131–144 (2002). doi:10.1016/S0378-5173(01)00891-2.
L.H. Emara, R.M. Badr, and A. Abd Elbary. Improving the dissolution and bioavailability of nifedipine using solid dispersions and solubilizers. Drug Dev. Ind. Pharm. 28:795–807 (2002). doi:10.1081/DDC-120005625.
A. Fahr, and X. Liu. Drug delivery strategies for poorly water-soluble drugs. Expert Opinion on Drug Delivery. 4:403–416 (2007). doi:10.1517/17425247.4.4.403.
V. Andronis, and G. Zografi. Crystal nucleation and growth of indomethacin polymorphs from the amorphous state. J. Non-Cryst. Solids. 271:236–248 (2000). doi:10.1016/S0022-3093(00)00107-1.
K.J. Crowley, and G. Zografi. The effect of low concentrations of molecularly dispersed poly(vinylpyrrolidone) on indomethacin crystallization from the amorphous state. Pharm Res. 20:1417–1422 (2003). doi:10.1023/A:1025706110520.
S. Vanhee, R. Koningsveld, H. Berghmans, K. Solc, and W.H. Stockmayer. Thermodynamic stability of immiscible polymer blends. Macromolecules. 33:3924–3931 (2000). doi:10.1021/ma9918102.
J.W. Barlow, and D.R. Paul. Polymer alloys. Annu. Rev. Mater. Sci. 11:299–319 (1981). doi:10.1146/annurev.ms.11.080181.001503.
M.M. Coleman, J.F. Graf, and P.C. Painter. Specific Interactions and the Miscibility of Polymer Blends. Technomic Publishing AG, Lancaster, Pennsylvania, 1991.
K.K. Chee. Thermodynamic study of glass transitions in miscible polymer blends. Polymer. 36:809–813 (1995). doi:10.1016/0032-3861(95)93112-Y.
E. Meaurio, E. Zuza, and J.R. Sarasua. Miscibility and specific interactions in blends of poly(L-lactide) with poly(vinylphenol). Macromolecules. 38:1207–1215 (2005). doi:10.1021/ma047818f.
P. Perrin, and R.E. Prudhomme. Miscibility behavior of PVC polymethacrylate blends—Temperature and composition analysis. Polymer. 32:1468–1473 (1991). doi:10.1016/0032-3861(91)90428-L.
Y. Park, B. Veytsman, M. Coleman, and P. Painter. The miscibility of hydrogen-bonded polymer blends: Two self-associating polymers. Macromolecules. 38:3703–3707 (2005). doi:10.1021/ma0473115.
S.B. Ahn, and H.M. Jeong. Phase behavior and hydrogen bonding in poly(ethylene-co-vinyl alcohol) poly(N-vinyl-2-pyrrolidone) blends. Korea Polym. J. 6:389–395 (1998).
S.L. Shamblin, E.Y. Huang, and G. Zografi. The effects of co-lyophilized polymeric additives on the glass transition temperature and crystallization of amorphous sucrose. J. Therm. Anal. 47:1567–1579 (1996). doi:10.1007/BF01992846.
P.J. Marsac, S.L. Shamblin, and L.S. Taylor. Theoretical and practical approaches for prediction of drug–polymer miscibility and solubility. Pharm. Res. 23:2417–2426 (2006). doi:10.1007/s11095-006-9063-9.
E.J. Moskala, D.F. Varnell, and M.M. Coleman. Concerning the miscibility of poly(vinyl phenol) blends—FTi.r. study. Polymer. 26:228–234 (1985). doi:10.1016/0032-3861(85)90034-5.
M.M. Coleman, and P.C. Painter. Hydrogen-bonded polymer blends. Prog. Polym. Sci. 20:1–59 (1995). doi:10.1016/0079-6700(94)00038-4.
R.S. Stein. Crystallization from polymer blends. Mater. Res. Soc. Symp. Proc. 321:531–542 (1994).
A.R. Kamdar, Y.S. Hu, P. Ansems, S.P. Chum, A. Hiltner, and E. Baer. Miscibility of propylene-ethylene copolymer blends. Macromolecules. 39:1496–1506 (2006). doi:10.1021/ma052214c.
D. Frezzotti, and G.P. Ravanetti. Evaluation of the Flory–Huggins interaction parameter for poly(styrene-co-acrylo-nitrile) and poly(methylmethacrylate) blend from enthalpy of mixing measurements. J. Therm. Anal. 41:1237–1243 (1994). doi:10.1007/BF02549918.
P.C. Painter, J.F. Graf, and M.M. Coleman. Effect of hydrogen-bonding on the enthalpy of mixing and the composition dependence of the glass-transition temperature in polymer blends. Macromolecules. 24:5630–5638 (1991). doi:10.1021/ma00020a023.
K. Khougaz, and S.D. Clas. Crystallization inhibition in solid dispersions of MK-0591 and poly(vinylpyrrolidone) polymers. J. Pharm. Sci. 89:1325–1334 (2000). doi:10.1002/1520-6017(200010)89:10<1325::AID-JPS10>3.0.CO;2-5.
H. Konno, and L.S. Taylor. Influence of different polymers on the crystallization tendency of molecularly dispersed amorphous felodipine. J. Pharm. Sci. 95:2692–2705 (2006). doi:10.1002/jps.20697.
L.S. Taylor, and G. Zografi. Spectroscopic characterization of interactions between PVP and indomethacin in amorphous molecular dispersions. Pharm. Res. 14:1691–1698 (1997). doi:10.1023/A:1012167410376.
L.S. Taylor, and G. Zografi. Sugar-polymer hydrogen bond interactions in lyophilized amorphous mixtures. J. Pharm. Sci. 87:1615–1621 (1998). doi:10.1021/js9800174.
P. Di Martino, E. Joiris, R. Gobetto, A. Masic, G.F. Palmieri, and S. Martelli. Ketoprofen-poly(vinylpyrrolidone) physical interaction. J. Cryst. Growth. 265:302–308 (2004). doi:10.1016/j.jcrysgro.2004.02.023.
P.J. Marsac, H. Konno, and L.S. Taylor. A comparison of the physical stability of amorphous felodipine and nifedipine systems. Pharm. Res. 23:2306–2316 (2006). doi:10.1007/s11095-006-9047-9.
S.I. Sandler. Chemical & Engineering Thermodynamics. Wiley, New York, 1999.
S.H. Yalkowsky. Solubility and Solubilization in Aqueous Media. Oxford University Press, New York, 1999.
P.J. Flory. Principles of Polymer Chemistry. Cornell University Press, Ithaca, 1953.
L. Mandelkern. Crystallization of polymers. McGraw-Hill, New York, 1964.
T. Nishi, and T.T. Wang. Melting-point depression and kinetic effects of cooling on crystallization in poly(vinylidene fluoride) poly(methyl methacrylate) mixtures. Macromolecules. 8:909–915 (1975). doi:10.1021/ma60048a040.
M. Rubinstein, and R.H. Colby. Polymer Physics. Oxford University Press, New York, 2003.
R.J. Young, and P.A. Lovell. Introduction to Polymers. Nelson Thornes, Cheltenham, UK, 1991.
S.L. Shamblin, X.L. Tang, L.Q. Chang, B.C. Hancock, and M.J. Pikal. Characterization of the time scales of molecular motion in pharmaceutically important glasses. J. Phys. Chem. B. 103:4113–4121 (1999). doi:10.1021/jp983964+.
B.C. Hancock, and M. Parks. What is the true solubility advantage for amorphous pharmaceuticals? Pharm. Res. 17:397–404 (2000). doi:10.1023/A:1007516718048.
X.L.C. Tang, M.J. Pikal, and L.S. Taylor. A spectroscopic investigation of hydrogen bond patterns in crystalline and amorphous phases in dihydropyridine calcium channel blockers. Pharm. Res. 19:477–483 (2002). doi:10.1023/A:1015147729564.
G.A. Jeffrey. An Introduction to Hydrogen Bonding. Oxford University Press, New York, 1997.
G. Van den Mooter, M. Wuyts, N. Blaton, R. Busson, P. Grobet, P. Augustijns, and R. Kinget. Physical stabilisation of amorphous ketoconazole in solid dispersions with polyvinylpyrrolidone K25. Eur. J. Pharm. Sci. 12:261–269 (2001). doi:10.1016/S0928-0987(00)00173-1.
D.J. Greenhalgh, A.C. Williams, P. Timmins, and P. York. Solubility parameters as predictors of miscibility in solid dispersions. J. Pharm. Sci. 88:1182–1190 (1999). doi:10.1021/js9900856.
K. Six, C. Leuner, J. Dressman, G. Verreck, J. Peeters, N. Blaton, P. Augustijns, R. Kinget, and G. Van den Mooter. Thermal properties of hot-stage extrudates of itraconazole and eudragit E100—Phase separation and polymorphism. J. Therm. Anal. Calorim. 68:591–601 (2002). doi:10.1023/A:1016056222881.
S.L. Shamblin, L.S. Taylor, and G. Zografi. Mixing behavior of colyophilized binary systems. J. Pharm. Sci. 87:694–701 (1998). doi:10.1021/JS9704801.
M. Yoshioka, B.C. Hancock, and G. Zografi. Inhibition of indomethacin crystallization in poly(vinylpyrrolidone) coprecipitates. J. Pharm. Sci. 84:983–986 (1995). doi:10.1002/jps.2600840814.
A. Saleki-Gerhardt, and G. Zografi. Nonisothermal and isothermal crystallization of sucrose from the amorphous state. Pharm. Res. 11:1166–1173 (1994). doi:10.1023/A:1018945117471.
P. Tong, and G. Zografi. A study of amorphous molecular dispersions of indomethacin and its sodium salt. J. Pharm. Sci. 90:1991–2004 (2001). doi:10.1002/jps.1150.
C.Q. Sun. A novel method for deriving true density of pharmaceutical solids including hydrates and water-containing powders. J. Pharm. Sci. 93:646–653 (2004). doi:10.1002/jps.10595.
K. Six, H. Berghmans, C. Leuner, J. Dressman, K. Van Werde, J. Mullens, L. Benoist, M. Thimon, L. Meublat, G. Verreck, J. Peeters, M. Brewster, and G. Van den Mooter. Characterization of solid dispersions of itraconazole and hydroxypropylmethylcellulose prepared by melt extrusion, part II. Pharm. Res. 20:1047–1054 (2003). doi:10.1023/A:1024414423779.
Acknowledgements
This work was supported in part by a fellowship from Merck Research Laboratories. AstraZeneca is acknowledged for financial support. Jared Baird is thanked for his help in measuring the solubility of the model compounds in 1-ethyl-2-pyrrolidone. Håkan Wikström is thanked for his help in measuring the solubility of sucrose using HPLC and Sheri Shamblin is thanked for providing heat capacity values for indomethacin and sucrose. Professors George Zografi, Rodolfo Pinal, Ken Morris, and Steve Byrn are acknowledged for their input. The PhRMA Foundation is acknowledged for a pre-doctoral fellowship to PJM. LST thanks AFPE/AACP for a New Investigator Award.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marsac, P.J., Li, T. & Taylor, L.S. Estimation of Drug–Polymer Miscibility and Solubility in Amorphous Solid Dispersions Using Experimentally Determined Interaction Parameters. Pharm Res 26, 139–151 (2009). https://doi.org/10.1007/s11095-008-9721-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-008-9721-1