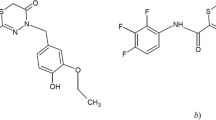
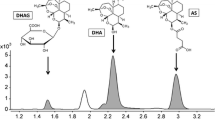
A method for assaying the antituberculosis drug thiozonide in plasma was developed and validated. Sample preparation consisted of precipitation of proteins with acetonitrile. Assay was by HPLC with a mass-selective detector. The method developed here was validated in terms of the following validation properties: selectivity, linearity, accuracy, precision, quantitative detection limit, sample transfer, and solution stability. The analytical range of the method was 1 – 1000 ng/ml thiozonide in plasma. This analytical range allows this method to be used for further studies of the pharmacokinetics of the innovative drug thiozonide.





Similar content being viewed by others
References
Decree “Approval of a Strategy for the Development of the Pharmaceutical Industry to 2020” [in Russian], Ministry of Industry and Trade, Moscow (2009); URL: http://www.minpromtorg.gov.ru/ministry/strategic/sectoral/7/utverzhdennayastrategiyafarma2020231009.pdf.
M. I. Perel’man, Phthysiatry. National Guidelines [in Russian], GEOTAR, Moscow (2007), pp. 10 – 22.
A. G. Khomenko, Handbook of Internal Diseases. Tuberculosis [in Russian], Meditsina, Moscow (1996), pp. 7 – 10.
Global Tuberculosis Report 2013 [in Russian], URL: http://www.who.int/tb/publications/globalreport/ru/.
G. R. Davies and E. L. Nuermberger, Tuberculosis, Supplement 1, 65 – 74 (2008).
L. S. Strachunskii, Yu. B. Belousov, and S. N. Kozlov (eds.), Practical Guidelines for Antiinfection Treatment [in Russian], NIIAKh SGMA, Smolensk (2007), pp. 286 – 290.
D. J. Guzman, X. Montes-Rincon andW. Ribon, “Research and Development of New Drugs Against Tuberculosis”, in: Tuberculosis = Current Issues in Diagnosis and Management, inTech, Bogota, Bucaramanga, Colombia (2013), Chapter 16.
G. D. Kapanadze, Author’s Abstract of Doctoral Thesis in Biological Sciences, Moscow (2011).
A. N. Mironov (ed.), Guidelines for Expert Evaluation of Drugs [in Russian], T. I, Grif i K, Moscow (2013).
Guidance for Industry: Bioanalytical Method Validation, U. S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evolution and Research (CDER), U. S. Government Printing Office, Washington, DC (2001).
Guideline on Validation of Bioanalytical Methods (draft), European Medicines Agency, Committee for Medicinal Products for Human Use, London (2009).
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiko-Farmatsevticheskii Zhurnal, Vol. 49, No. 3, pp. 46 – 10, March, 2015.
Rights and permissions
About this article
Cite this article
Ramenskaya, G.V., Shokhin, I.E., Men’shikova, L.A. et al. Development and Validation of a Method for Assay of the Original Antituberculosis Agent Thiozonide in Plasma for Pharmacokinetic Studies. Pharm Chem J 49, 199–202 (2015). https://doi.org/10.1007/s11094-015-1254-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11094-015-1254-4